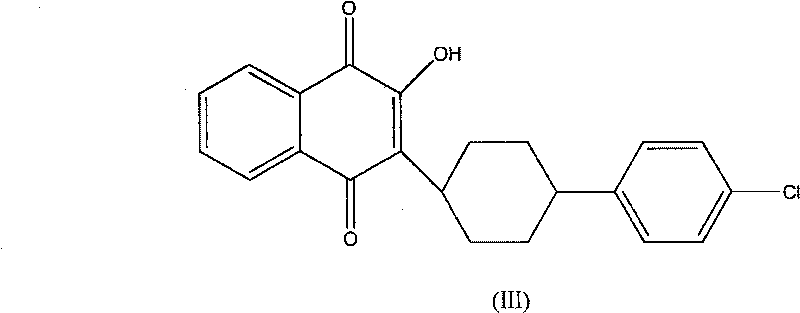
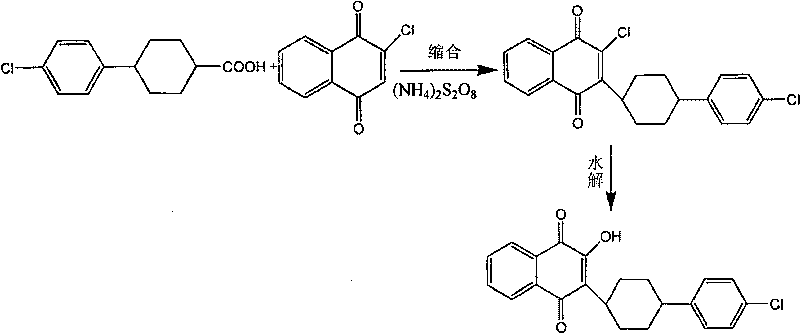
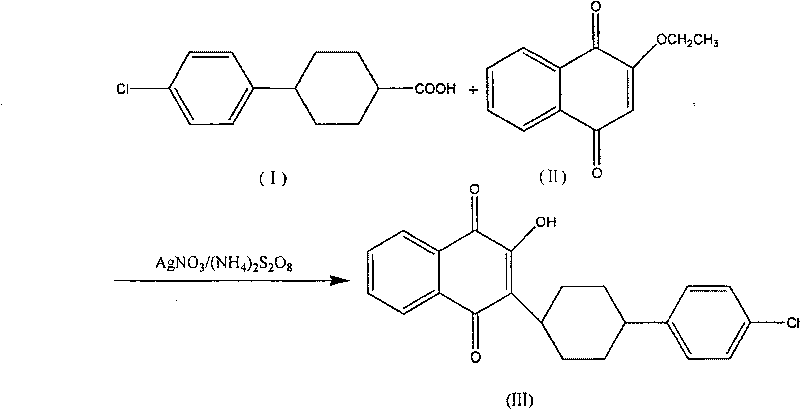
Technique for synthesizing atovaquone
A synthesis process, a technology of atovaquone, is applied in the technical field of chemical synthesis of drugs, can solve the problems of slow reaction speed, low purity of pure atovaquone, long reaction time, etc., and achieves low cost, high purity, The effect of saving synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 2-Ethoxy-1,4-naphthoquinone (II) (4.30g, 0.02mol), 4-(4-chlorophenyl)cyclohexyl-1-carboxylic acid (I) (4.9g, 0.02mol), Silver nitrate (1.05g, 0.0062mol), acetonitrile 40ml joins in the 500ml four-necked flask, is heated to reflux while stirring with the rotating speed of 300rpm, adds dropwise 80ml ammonium persulfate aqueous solution (12.0g, 0.0525mol) in 1 hour, Reflux and stir for 3 hours, then cool to 0°C, maintain for 30 minutes, filter to obtain a yellow powdery solid, dissolve the crystallized product with 30ml of chloroform, filter off the insoluble matter, distill off the chloroform under reduced pressure, and recrystallize with 40ml of acetonitrile to obtain Atovaquone (III) yellow needle crystal (0.6g), melting point 216°C-219°C (melting point of atovaquone in reference literature is 216°C-219°C), yield 7.8%.
Embodiment 2
[0023] 2-Ethoxy-1,4-naphthoquinone (II) (10.20g, 0.05mol), 4-(4-chlorophenyl)cyclohexyl-1-carboxylic acid (I) (11.94g, 0.05mol), Silver nitrate (1.05g, 0.025mol), acetonitrile 150ml joins in the 500ml four-necked flask, is heated to reflux while stirring with the rotating speed of 200rpm, adds dropwise 160ml ammonium persulfate aqueous solution (34.2g, 0.15mol) in 50min, reflux After stirring for 4 hours, cool to 0°C, maintain for 30 minutes, filter to obtain a yellow powdery solid, dissolve the crystallized product with 80ml of chloroform, filter off the insoluble matter, distill off the chloroform under reduced pressure, and recrystallize with 90ml of acetonitrile to obtain Tovaquinone (III) yellow needle-like crystals (1.38g), yield 7.5%.
Embodiment 3
[0025] The synthesis process steps and process conditions are the same as in Example 1, except that the amount of ammonium persulfate aqueous solution added is: 80ml ammonium persulfate aqueous solution (9.1g, 0.04mol), and the final yield is 7.65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com