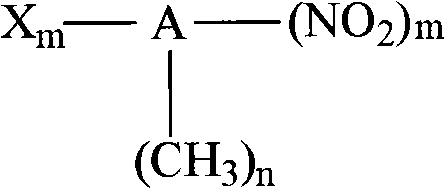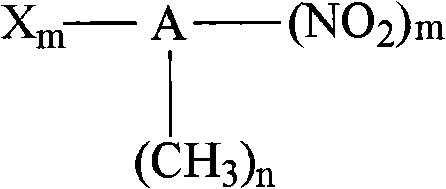Catalytic hydrogenation method for preparing halogenated aromatic amine from halogenated arene nitro compounds
A technology of nitro compounds and halogenated aromatics, which is applied in the field of catalytic hydrogenation of nitro compounds of halogenated aromatics to prepare halogenated aromatic amines, which can solve problems such as environmental pollution and increase the burden of product separation procedures, and reduce costs and production accidents risks, effects of decontamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 ortho-chloronitrobenzene (o-CNB) hydrogenation
[0021] Add 1.5g o-CNB, 150mg reduced Ni / TiO to a 50ml stainless steel reactor 2 -I catalyst, Ni / TiO 2 -I catalyst impregnated TiO with nickel nitrate solution 2 The powder is prepared, and the mass content of Ni in the catalyst is 15%. Tighten the reactor, purging with high-purity nitrogen for 3 minutes at room temperature, and then using 2MPa H 2 Replace 2 times to get rid of the air in the reactor. The reaction kettle was preheated in a constant temperature water bath at 35°C for 20min, and then 4MPa H 2 , 10.5MPa CO 2 , start stirring, react at 35°C for 50 min, and analyze the product by gas chromatography after being diluted with ethanol. The conversion of o-CNB was 82%, and the selectivity of o-chloroaniline was 99.8%.
Embodiment 2
[0026] Embodiment 2 m-chloronitrobenzene (m-CNB) hydrogenation
[0027] Add 1.5g m-CNB and 150mg catalyst in a 50ml reactor, the catalyst is the same as in Example 1. Tighten the reaction kettle, purging with high-purity nitrogen, H at room temperature 2 After the replacement, the reactor was preheated in a constant temperature water bath at 35°C for 20 minutes, and then 4MPa H 2 , 11MPa CO 2 , start stirring, react at 35°C for 80 min, and analyze the product by gas chromatography after being diluted with ether. The conversion rate of m-CNB was 100%, and the selectivity of m-chloroaniline was 99.5%.
Embodiment 3
[0028] Embodiment 3 p-chloronitrobenzene (p-CNB) hydrogenation
[0029] Add 1.5g p-CNB, 150mg catalyst in 50ml reactor, catalyst is the same as embodiment 1. Tighten the reaction kettle, purging with high-purity nitrogen, H at room temperature 2 After the replacement, the reactor was preheated in a constant temperature water bath at 35°C for 20 minutes, and then 4MPaH 2 , 11MPa CO 2 , start stirring, react at 35°C for 30min, and analyze the product by gas chromatography. The conversion rate of p-CNB was 50.5%, and the selectivity to p-chloroaniline was 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com