Method for producing high-purity mezlocillin sodium and powder injection thereof
A technology of mezlocillin sodium powder and mezlocillin sodium, which is applied in the field of medicine, can solve the problems of poor stability, poor clarity, and low purity, and achieve the effects of improving stability, improving purity, and improving clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
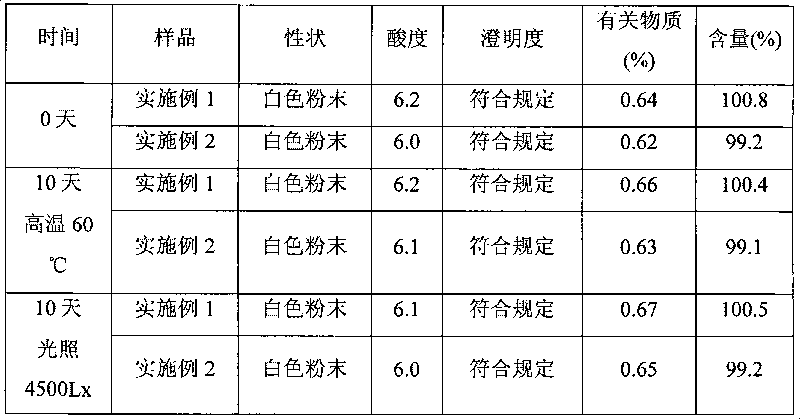
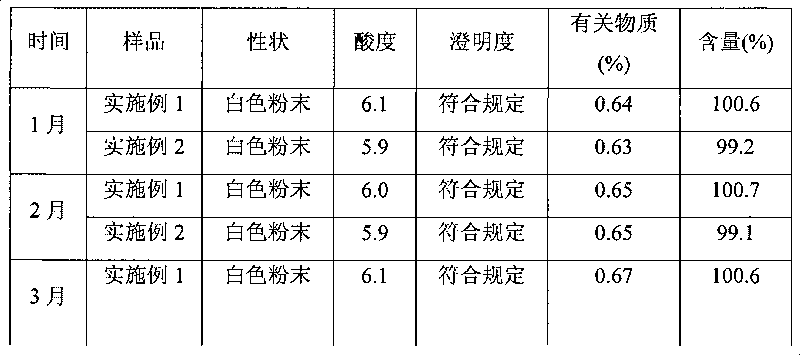
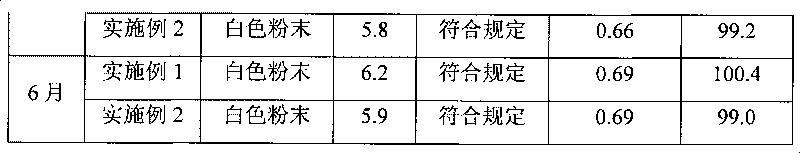
[0050] Stir and dissolve mezlocillin acid in purified water, add 2 mol / L sodium hydroxide solution to adjust the pH of the solution to 8.7, then adsorb it with D1300 macroporous resin, and elute it with distilled water until there is almost no mezlocillin sodium in the eluent. Collect the eluate, concentrate the eluate by evaporation under reduced pressure, and use SephadexC 15 Carry out column chromatography on a cross-linked Sephadex column as a filler, elute with 0.05mol / L ammonium formate aqueous solution, the elution temperature is 15°C, collect the part with an integral area ratio of 99% or more detected by HPLC, and add a solution volume of 0.2 % (g / ml) activated carbon, decolorized at room temperature for 30 minutes, sterilized by filtration with a 0.22um microporous membrane, and freeze-dried to obtain mezlocillin sodium with a purity of 99.5% and a yield of 87.8%.
[0051] After the above-mentioned mezlocillin sodium pure product is freeze-dried, crushed through a 60...
Embodiment 2
[0053] Stir and dissolve mezlocillin acid in purified water, add 10% sodium carbonate solution to adjust the pH of the solution to 9.0, adsorb with D1300 macroporous resin, and elute with distilled water until there is almost no mezlocillin sodium in the eluent. Collect the eluate, concentrate the eluate by evaporation under reduced pressure, and use SephadexC 25 Carry out column chromatography on a cross-linked Sephadex column as a filler, elute with 0.1mol / L ammonium acetate aqueous solution, and the elution temperature is 20°C, collect the part with an integral area ratio of 99% or more detected by HPLC, and add a solution volume of 0.2 % (g / ml) activated carbon, decolorized at room temperature for 30 minutes, sterilized by filtration with a 0.22um microporous membrane, and freeze-dried to obtain mezlocillin sodium with a purity of 99.6% and a yield of 88.2%.
[0054] After the above-mentioned mezlocillin sodium pure product is freeze-dried, pulverize through a 100-mesh sie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com