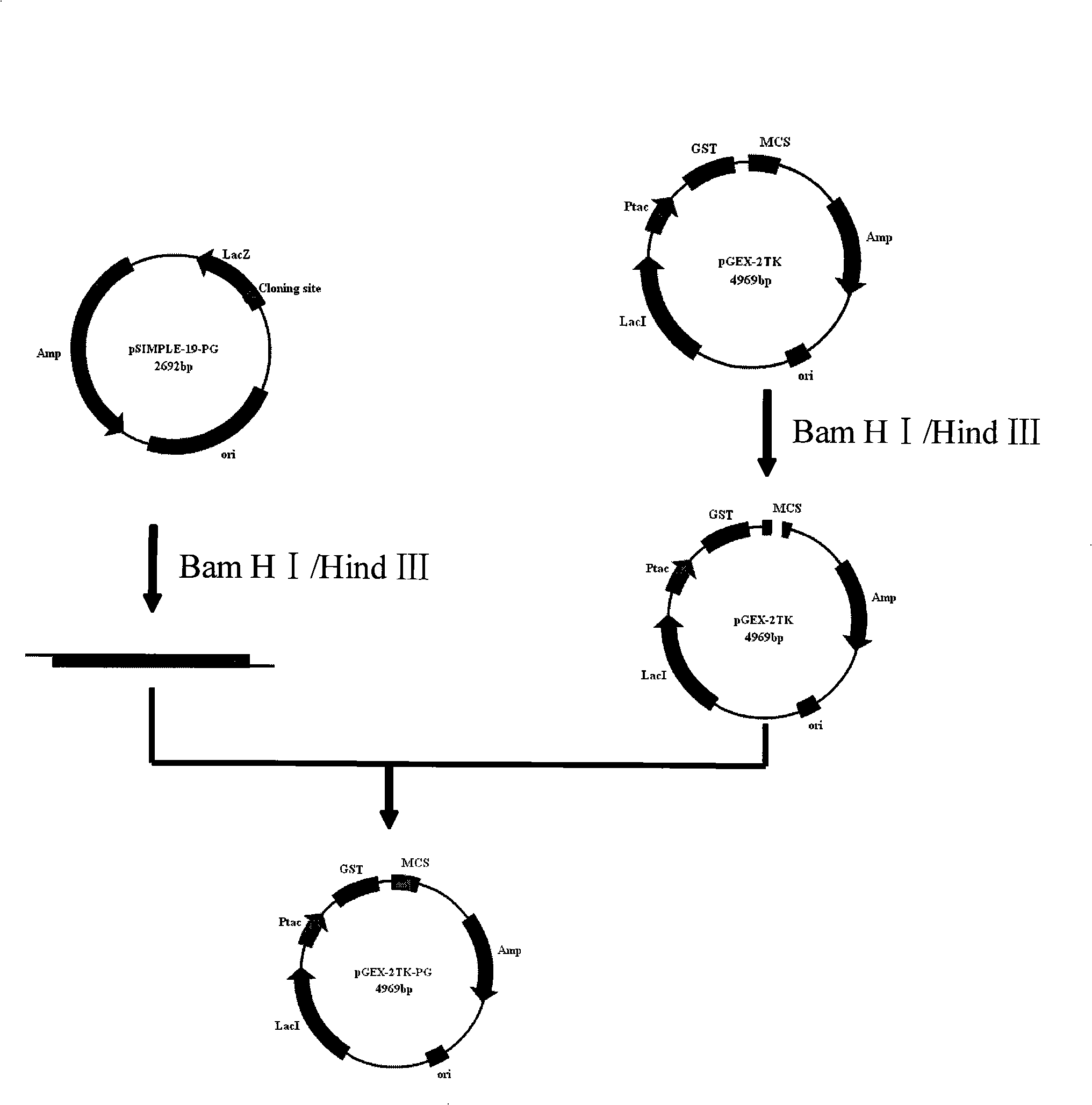
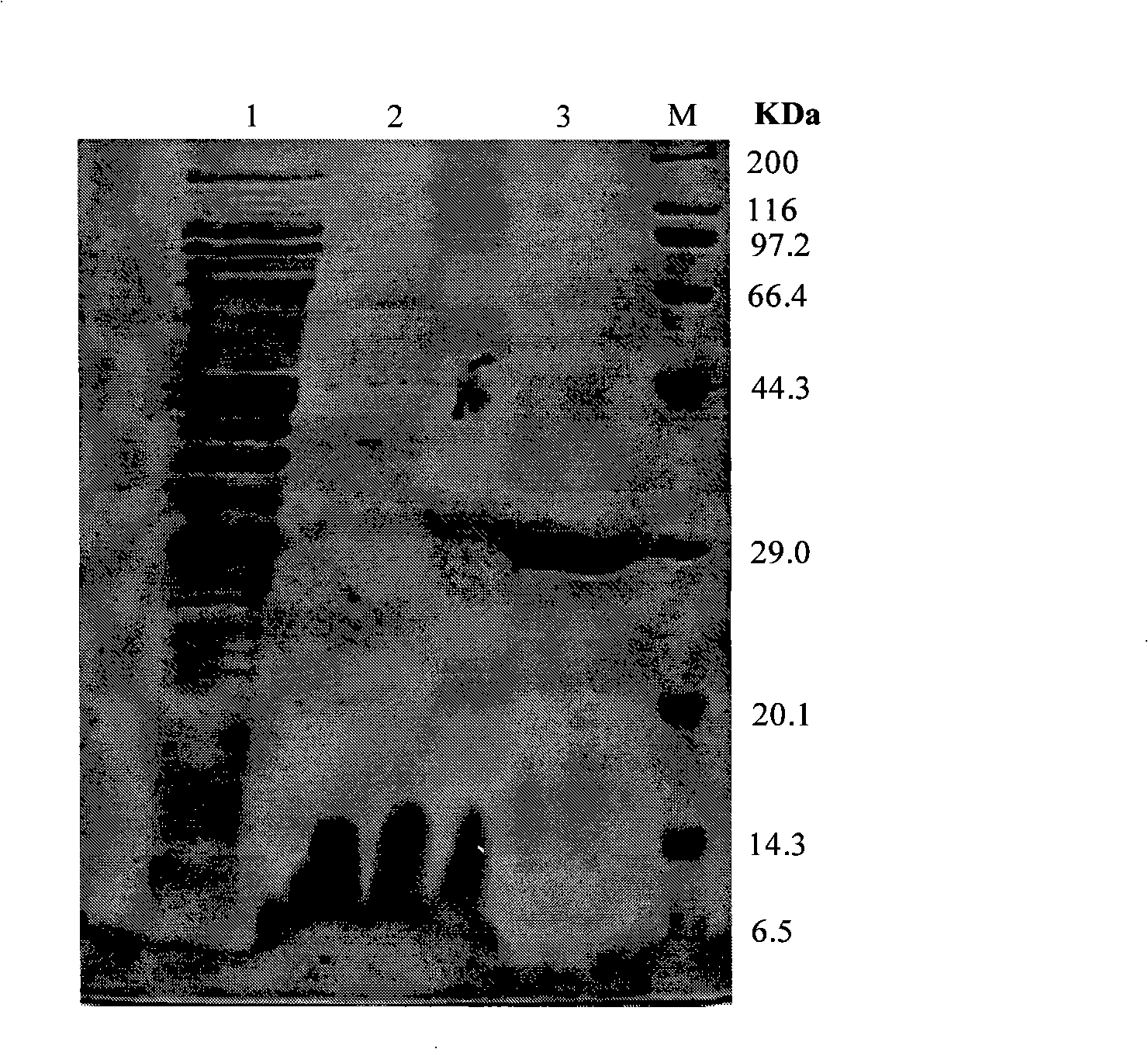
Recombination pig origin antibiotic peptide PG4 and its biological synthesis method and application
A synthesis method and antibacterial peptide technology are applied in the field of genetic recombinant pig-derived antibacterial peptides and their biosynthesis, which can solve the problems of high price of antibacterial peptides and low natural yield of antibacterial peptides, and achieve easy amplification, easy operation and high expression efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Cloning of a recombinant pig-derived antimicrobial peptide PG4 gene
[0037] (1) Design and synthesize two oligonucleotides: PG4-1 and PG4-2, and then obtain a double-helix DNA fragment through annealing and renaturation, and its nucleotide sequences are respectively:
[0038] PG4-1:
[0039] 5'-ATTCAGGGATCCATGCGCGGTGGCCGTCTGTGCTATTGTCGCGGTTGGATCTGCTTCTGTGTGGGTCGTTAAAAGCTTAATTCG-3';
[0040] PG4-2:
[0041] 5'-CGAATTAAGCTTTTAACGACCCACACAGAAGCAGATCCAACCGCGACAATAGCACAGACGGCCACCGCGCATGGATCCCTGAAT-3'.
[0042] Two oligonucleotide fragments, PG4-1 and PG4-2, were chemically synthesized using Escherichia coli preferred codons. The double-helix DNA obtained by annealing PG4-1 and PG4-2 corresponds to the amino acid sequence of SEQ ID NO4:Ile- Gln-Gly-Ser-Met-Arg-Gly-Gly-Arg-Leu-Cys-Tyr-Cys-Arg-Gly-Trp-Ile-Cys-Phe-Cys-Val-Gly-Arg-Terminator-Lys-Leu -Asn-Ser.
[0043]At both ends of the two oligonucleotide fragments, BamH I and HindIII restriction endonuclease sites were...
Embodiment 2
[0066] Antibacterial Test of Recombinant Antimicrobial Peptide PG4
[0067] 1. Recombinant antimicrobial peptide PG4 anti-Escherichia coli E.coli test:
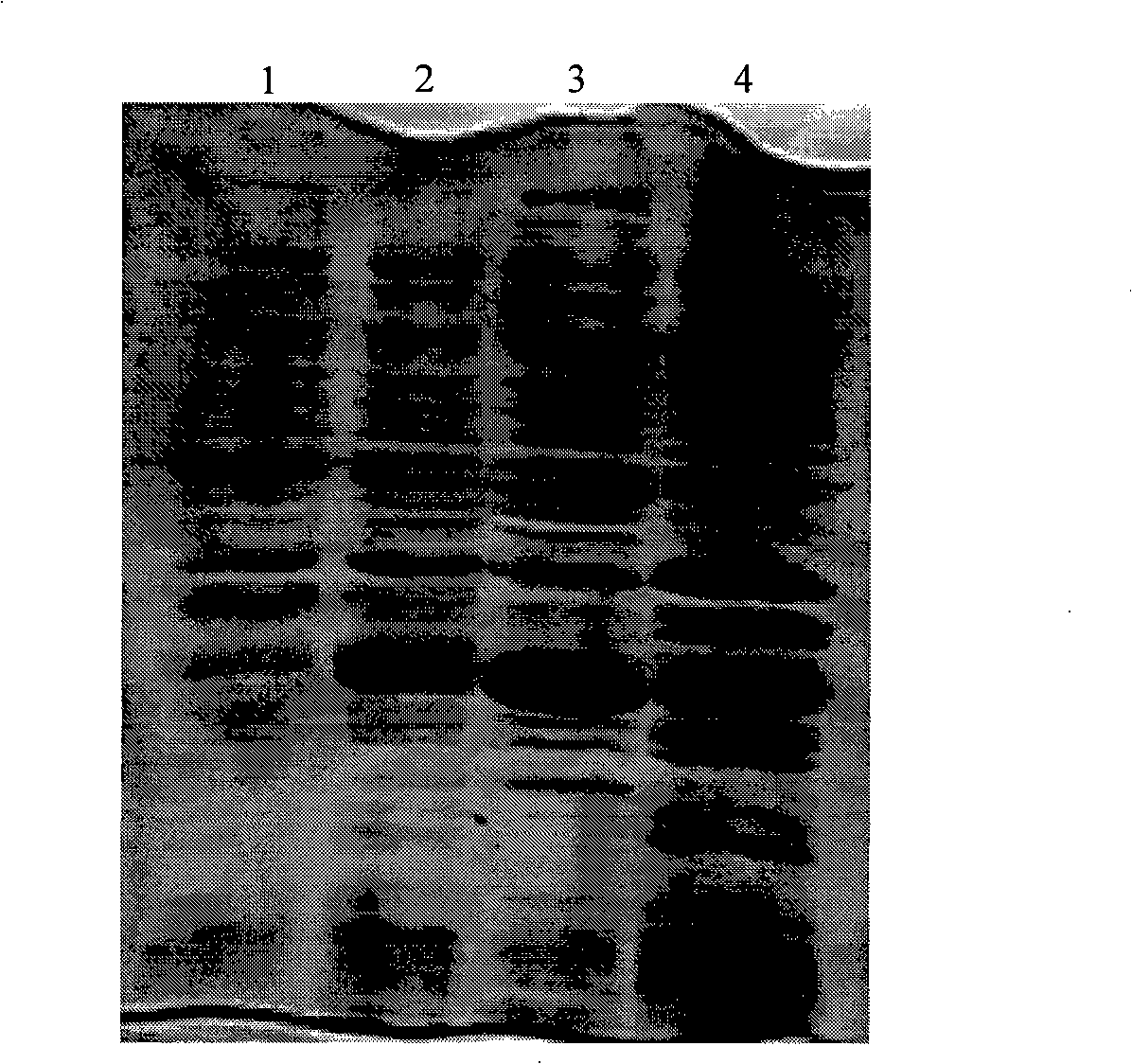
[0068] (1) Two pieces of filter paper with a diameter of 1 cm were taken, sterilized by ultraviolet irradiation (irradiation time was 30 minutes), and infiltrated with sterile water and recombinant antimicrobial peptide PG4 solution (0.1 mg / mL) respectively. exist Figure 4 Among them, sample 1 and sample 2 in turn;
[0069] (2) Put the above two samples into sterile cell culture dishes respectively, and then add 50 μL E. coli culture solution (10 6 CFU / mL), and cultivated in a 37°C incubator for 2 hours;
[0070] (3) Add 1 mL of phosphate buffer solution to each petri dish, and clean it with ultrasonic waves (64kHz, 2 minutes), then take 50 μL of gradient dilution, add 50 μL of bacterial buffer solution of different dilutions to the culture dish, and use a coating Spread the bacterial solution evenly on the solid agar me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com