Decellularization and recellularization of organs and tissues
A decellularization, organ technology, applied in the field of organs and tissues, which can solve the problem of no ideal matrix structure, no display of organs or tissues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 - Preparation of solid organs for decellularization
[0058] To avoid postmortem thrombus formation, donor rats were systemically heparinized with a dose of 400 U of heparin / kg of donor. After heparinization, carefully dissect the heart and adjacent great vessels.
[0059] The heart was placed in physiological saline solution (0.9%) containing heparin (2000 U / ml) and stored at 5°C until further processing. Under sterile conditions, separate the connective tissue from the heart and great vessels. The inferior vena cava and left and right pulmonary veins distal to the right and left atria were ligated with non-absorbable monofilament ligatures.
Embodiment 2
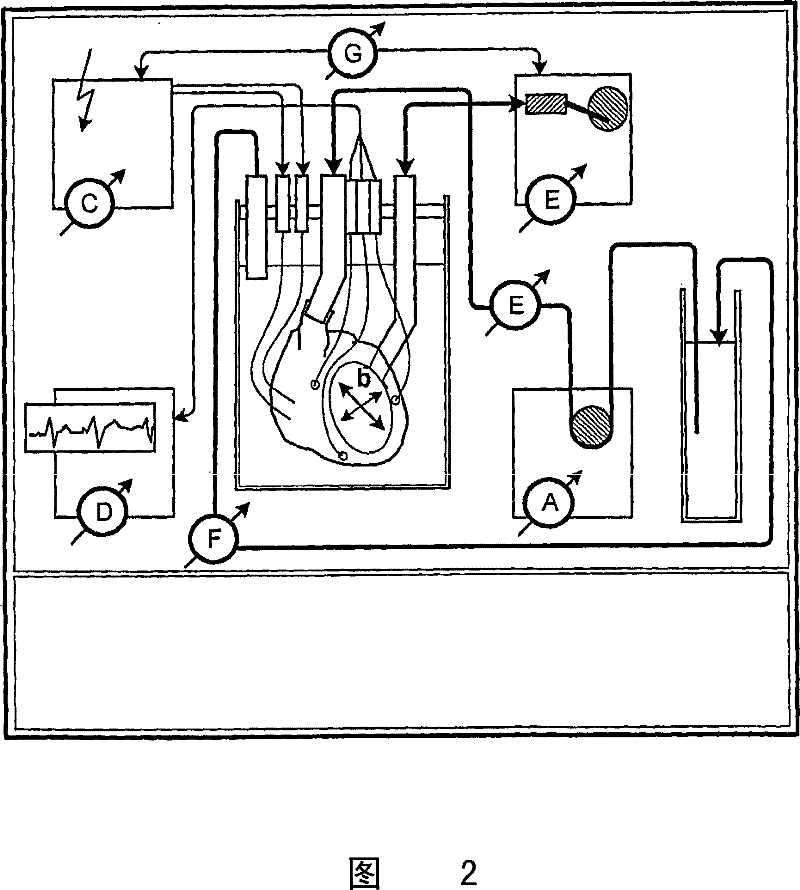
[0060] Example 2 - Cannulation and perfusion of solid organs
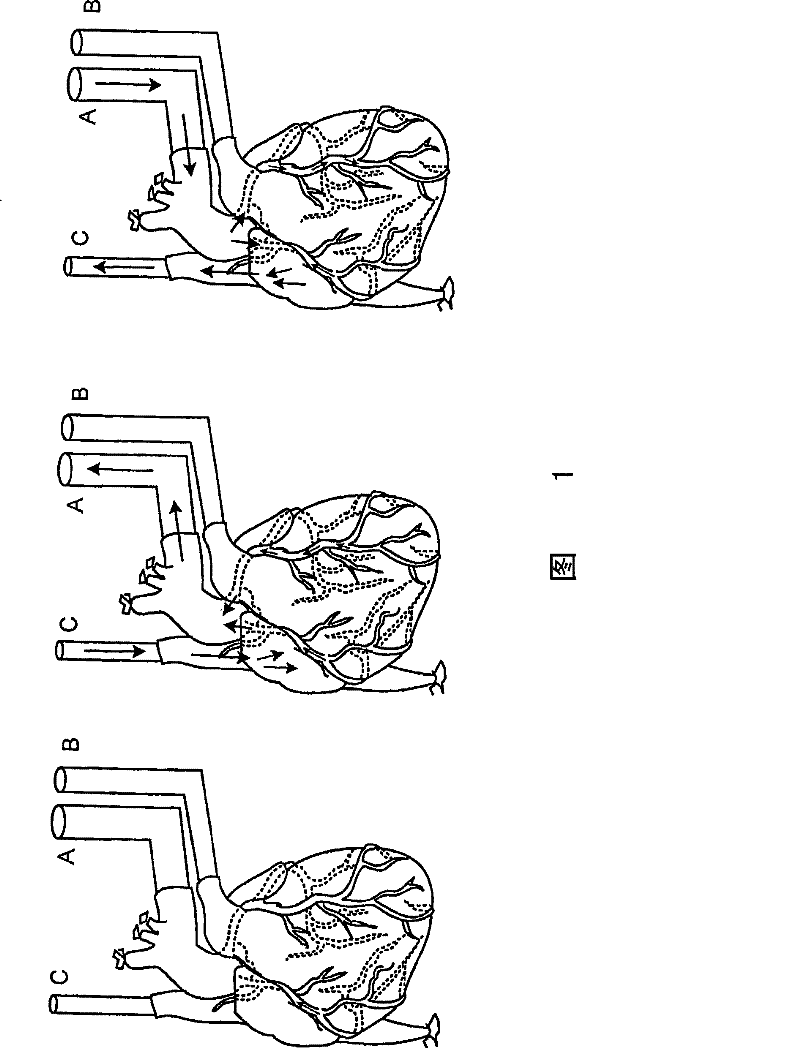
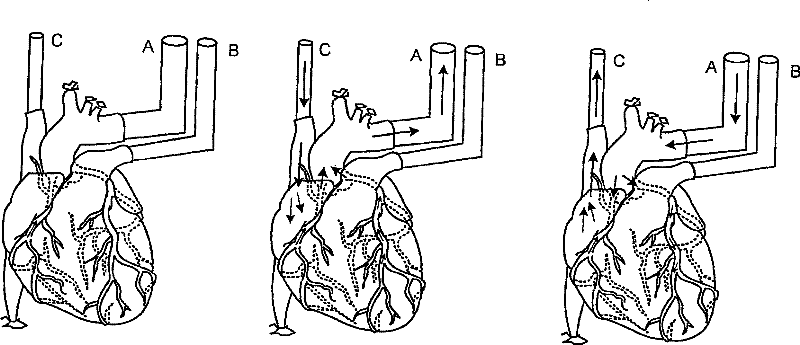
[0061] The heart was fixed on a decellularization device for perfusion ( figure 1 ). Cannulation of the descending thoracic artery for retrograde coronary (arterial) perfusion ( figure 1 , intubation A). Ligate branches of the thoracic arteries (eg, brachiocephalic, left common carotid, left subclavian). Cannulate the pulmonary artery before it divides into left and right pulmonary arteries ( figure 1 , intubation B). Cannulation of the superior vena cava ( figure 1 , intubation C). This configuration enables both retrograde and antegrade coronary (arterial) perfusion.
[0062] Perfusion from the coronary arteries through the capillary bed to the coronary venous system to the right atrium and superior vena cava (C) occurs when positive pressure is applied to the aortic cannula (A). Perfusion from the right atrium, coronary sinus, and coronary veins through the capillary bed to the coronary and aortic cann...
Embodiment 3
[0063] Example 3 - Decellularization
[0064] After fixation of the heart in the decellularization device, antegrade perfusion was initiated with cold heparinized calcium-depleted phosphate buffer containing 1-5 mmol adenosine / L infusion to reestablish continuous coronary (arterial) flow. Coronary (arterial) flow was assessed by measuring coronary (arterial) infusion pressure and flow, and coronary resistance was calculated. After 15 minutes of steady coronal flow, detergent-based decellularization was initiated.
[0065] The specific processing method is as follows. However, in brief, the heart is perfused antegradely with detergent. After perfusion, flush the heart retrogradely with buffer (eg, PBS). Then, the hearts were perfused with PBS containing antibiotics, and then with PBS containing DNase I. The heart was then perfused with 1% benzalkonium chloride to reduce and prevent future microbial contamination, and the organ was perfused with PBS to remove any residual ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com