Method for preparing bonding type cyclodextrin stationary phase with click chemistry reaction
A technology of cyclodextrin and stationary phase, which is applied in the field of chromatographic stationary phase, can solve the problems of harsh reaction conditions, difficult operation, complex preparation process of bonded cyclodextrin stationary phase, etc., achieves simple preparation method, avoids side reactions, Effect of High Column Efficiency and Separation Selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
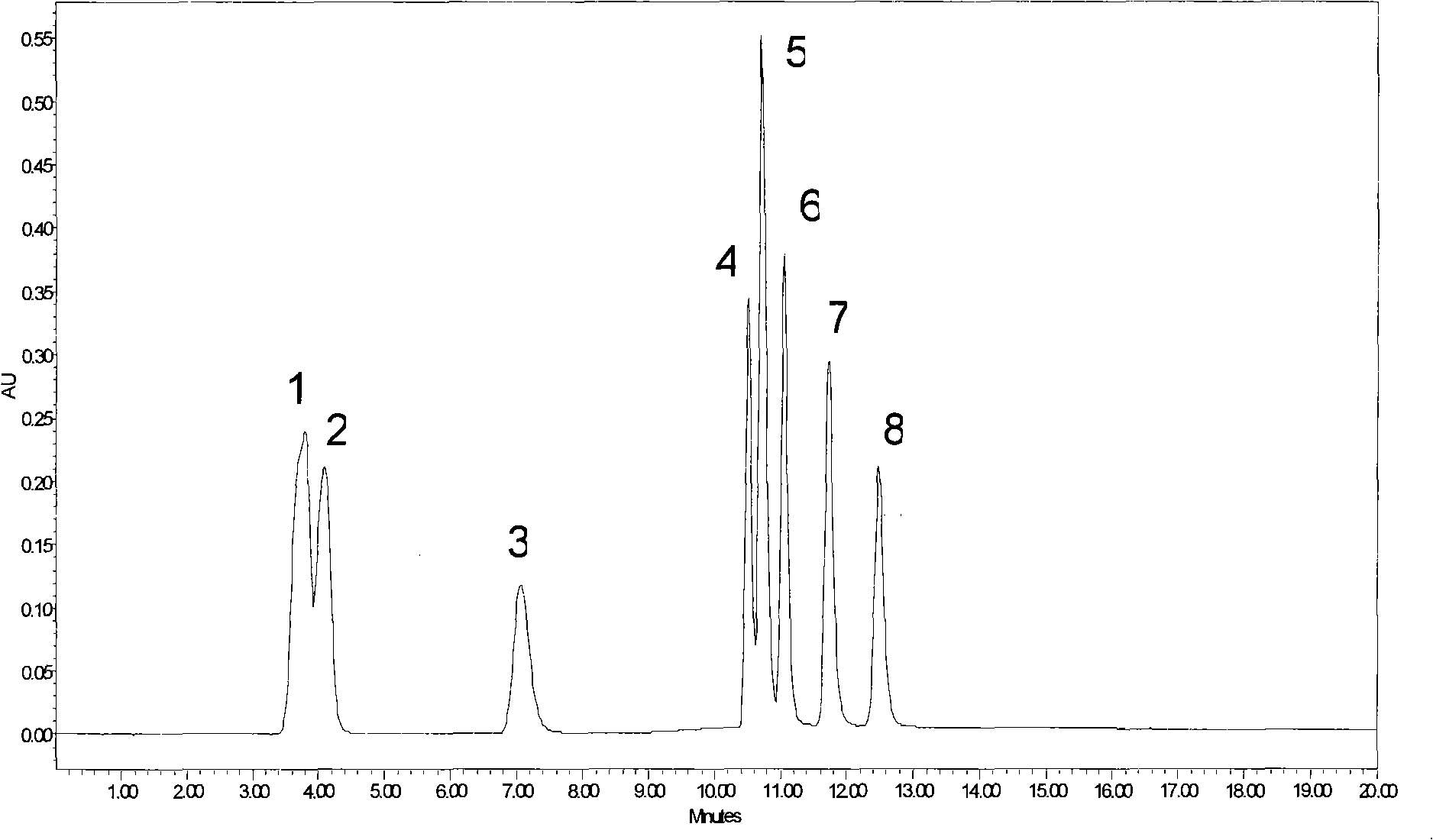
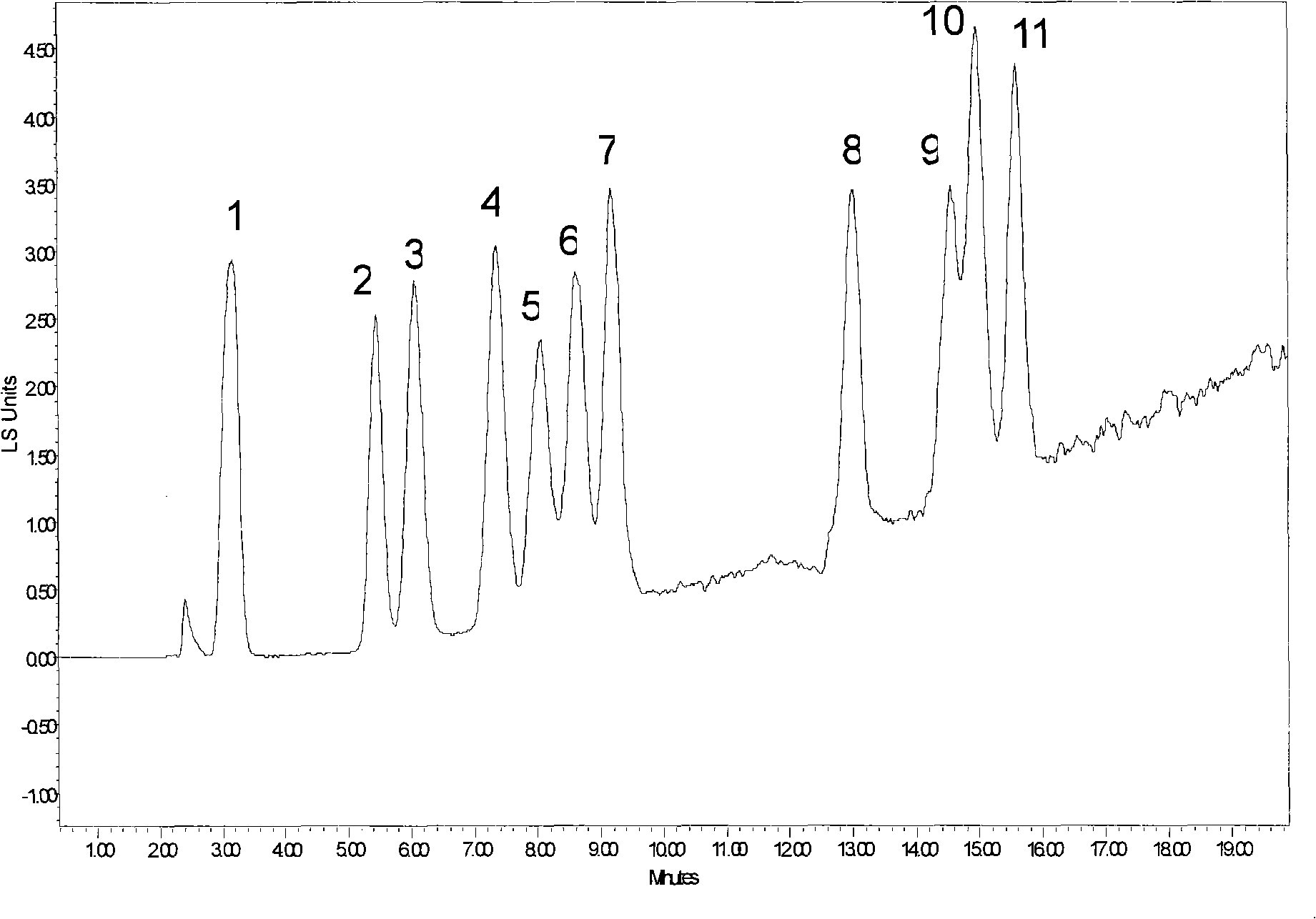
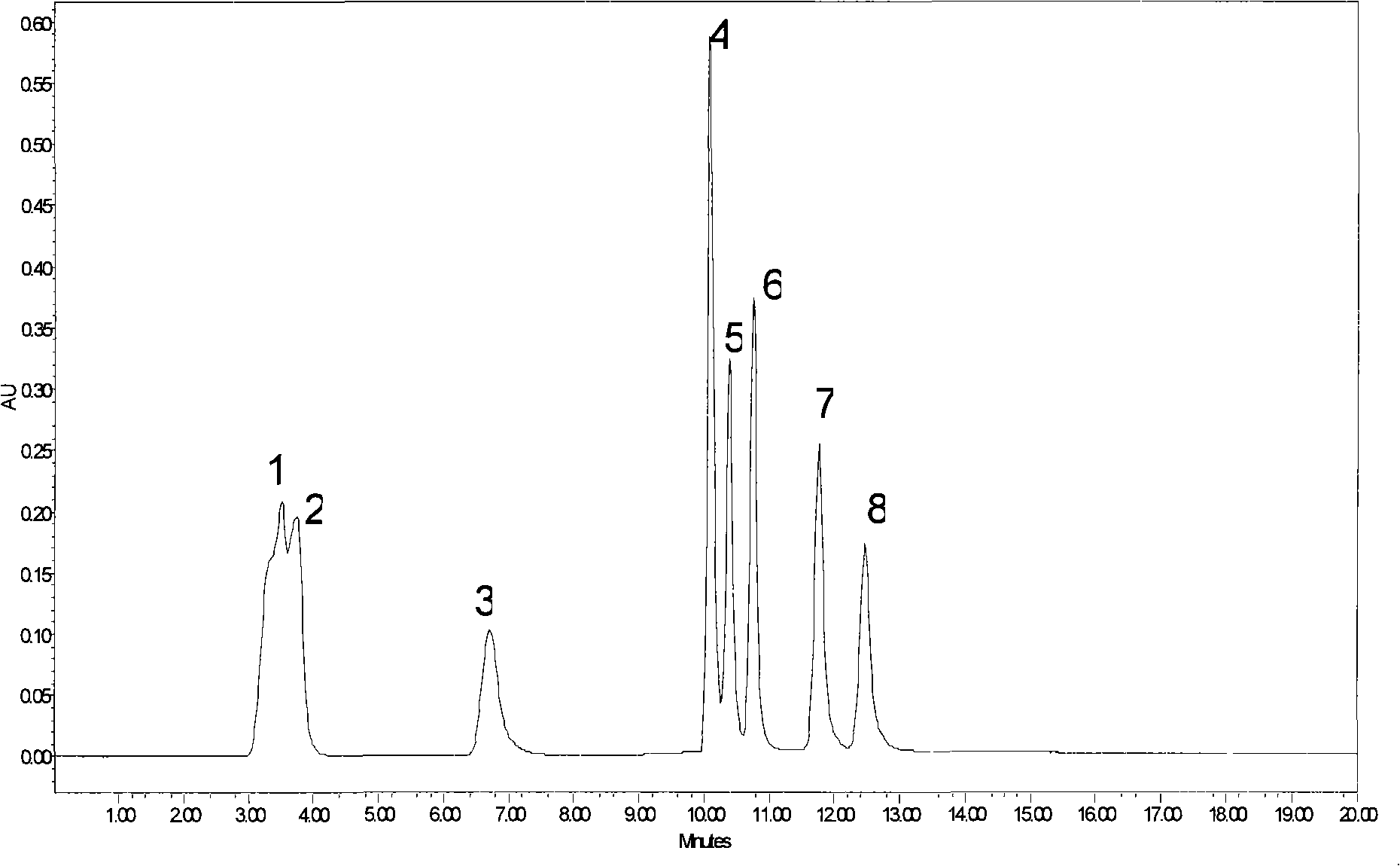
Image
Examples
Embodiment 1
[0049] In a 1000ml three-necked flask, add 500ml of anhydrous DMF, 45mmol (about 10ml) of 3-chloropropyltriethoxysilane, 60mmol (about 4.0g) of sodium azide, and then add 3.0mmol of sodium iodide (about 0.5g) As a catalyst, react at 80°C for 24h, then add 20g of spherical silica gel particles with a particle size of 5μm, react at 80°C for another 24h, and then wash with dichloromethane, ethanol, water, and acetone twice, each time 200ml, 60 °C and vacuum-dried for 12 hours to obtain (3-propyl)azido silica gel.
[0050] In a 50ml three-neck flask under the protection of nitrogen, 15ml of propargylamine was added, and then 6g of mono-6-p-toluenesulfonyl-β-cyclodextrin was added, and heated to reflux for 12 hours. After cooling to room temperature under nitrogen protection, the reaction system was poured into acetonitrile to precipitate a solid. The solid was recrystallized from methanol to obtain the product mono-6-deoxy-N-propargylamino-β-cyclodextrin.
[0051] Take 2.5g of d...
Embodiment 2
[0054] Add 500ml of anhydrous toluene, 120mmol (about 30ml) of 3-isocyanatotriethoxysilane, 160mmol of propargyl amine (about 9g) into a 1000ml three-necked flask, react at 80°C for 12h, and then add Spherical silica gel particles 40g were reacted at 80°C for 48h, then washed twice with dichloromethane, ethanol, water, and acetone, 200ml each time, and vacuum-dried at 60°C for 12 hours to obtain alkynyl silica gel derivatives.
[0055] A 50ml three-neck flask was under nitrogen protection, 15ml of DMF was added, and then 10g of mono-6-p-toluenesulfonyl-β-cyclodextrin was added, and heated to 65°C for 24 hours. The solvent DMF was concentrated to 5ml, poured into acetone to precipitate a solid. The solid is recrystallized with water to obtain the product mono-6-deoxy-β-azidocyclodextrin.
[0056] Take 2.5g of dried alkynyl silica gel derivatives, add 60ml of water, 60ml of ethanol, 4g of mono-6-deoxy-azido-cyclodextrin into the reactor, then add 0.05g of copper sulfate and 0.1...
Embodiment 3
[0059] The difference from Example 1 is that mono-6-p-toluenesulfonyl-α-cyclodextrin is used instead of mono-6-p-toluenesulfonyl-β-cyclodextrin, according to the synthesis of Example 1 Steps can get another cyclodextrin bonded stationary phase, the structure of which is:
[0060]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com