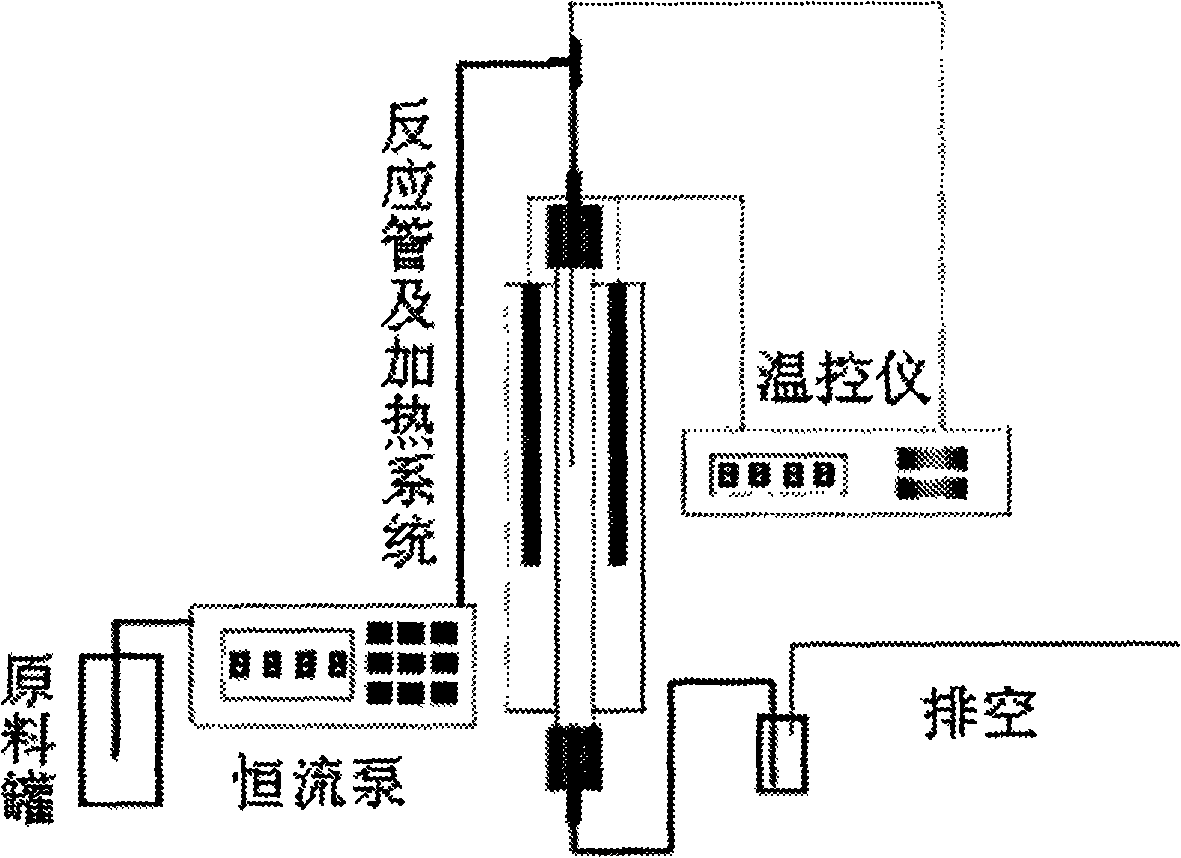
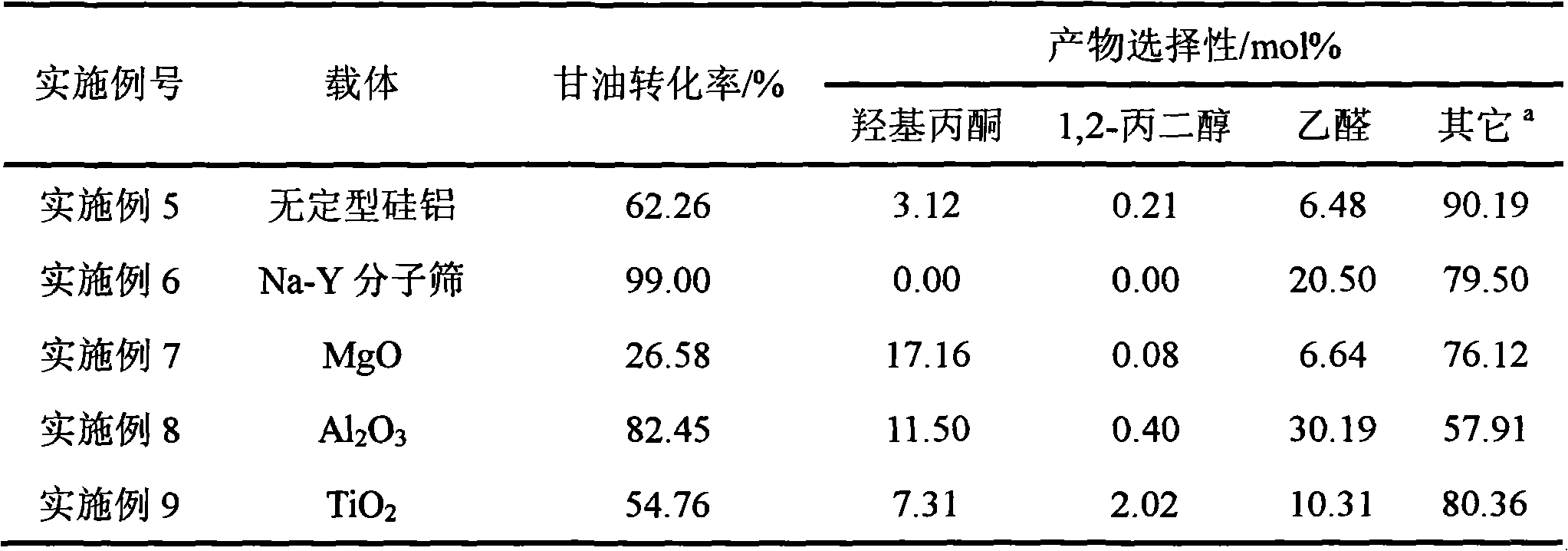
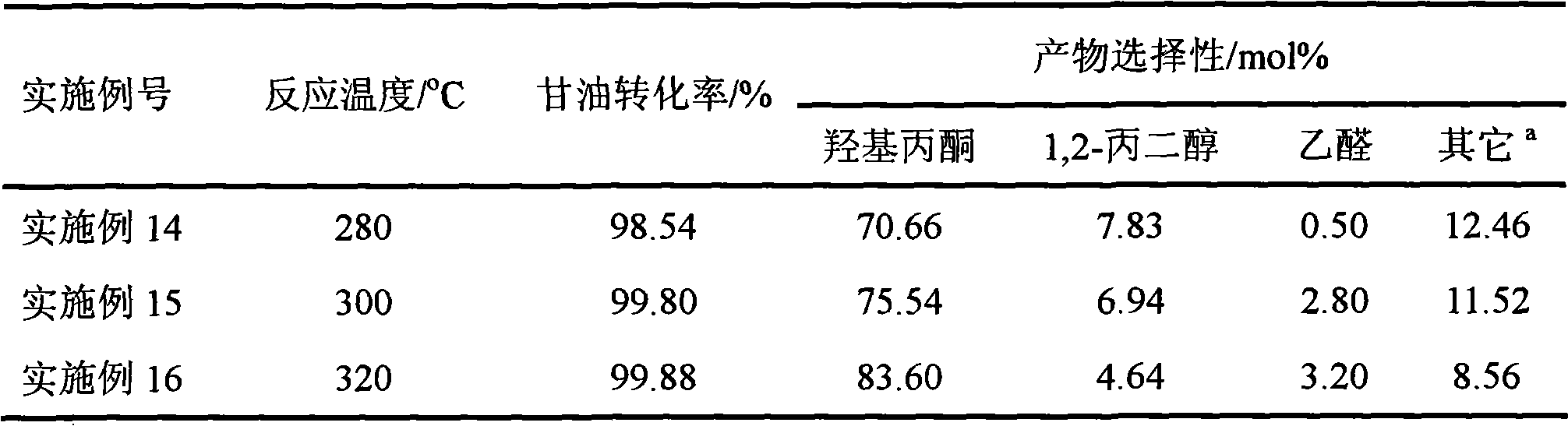
Reaction for preparing hydroxyacetone by selectively dewatering natural glycerol and catalyst
A technology of natural glycerol and hydroxyacetone, applied in physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, preparation of carbon-based compounds, etc., can solve problems such as easy curing and deactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 20g of silicon dioxide was calcined at 600°C for 6 hours, cooled to room temperature, pressed into tablets and granulated, and the catalyst of 20-30 meshes was screened for use.
[0015] A proper amount of catalyst is filled in the quartz reaction tube of the fixed bed reactor, and 20-30 mesh quartz sand is filled in the upper part of the catalyst bed and the lower part of the reactor space. Hydrogen gas was introduced into the reactor, and the catalyst was reduced for 6 hours under the conditions of a pressure of 0.01 MPa and a temperature of 300°C. After the reaction tube is cooled to room temperature, 40% glycerol aqueous solution is fed into the reactor with a constant flow pump to carry out selective dehydration of glycerin, and the LHSV is 0.1h -1 , The dehydration reaction temperature is 310°C. The result of the reaction is as follows:
[0016] Glycerin conversion rate is 10.50%; Product molar selectivity is: hydroxyacetone 3.33%, 1,2-propanediol 2.35%, acetald...
Embodiment 2
[0018] Weigh 5g of copper nitrate trihydrate, 15g of silicon dioxide and 50g of deionized water in a beaker and mix evenly, stir at room temperature for 6 hours, remove the liquid and dry at 120°C, bake at 600°C for 6 hours, cool to room temperature, repeat the above Cu / SiO with 20% CuO content was prepared by impregnation loading and firing process 3 times 2 catalyst. The catalyst is tableted and granulated, and the granules with 20-30 meshes are screened for the reaction of glycerin dehydration to produce hydroxyacetone.
[0019] Reaction process condition is with embodiment 1. The result of the reaction is as follows:
[0020] Glycerin conversion rate is 51.64%; Product molar selectivity is: hydroxyacetone 38.18%, 1,2-propanediol 1.69%, acetaldehyde 6.78%, and other components (mainly acetic acid, dihydrofuran, dihydroxyfuran, glycerol acetate esters, etc.) 53.35%.
Embodiment 3
[0022] Weigh 9g of ferric chloride hexahydrate, 15g of silicon dioxide and 50g of deionized water in a beaker and mix evenly, stir at room temperature for 6 hours, remove the liquid and dry at 120°C overnight, roast at 600°C for 6 hours, and cool to room temperature , repeat the above impregnation loading and roasting process twice to obtain Fe 2 o 3 28% Fe / SiO 2 catalyst. Tablet granulation, screening of 20-30 mesh catalysts for use.
[0023] Reaction process condition is with embodiment 1. The result of the reaction is as follows:
[0024]Glycerin conversion rate is 16.90%; Product molar selectivity is: hydroxyacetone 1.92%, 1,2-propanediol 1.71%, acetaldehyde 7.10%, and other components (mainly acetic acid, dihydrofuran, dihydroxyfuran, glycerol acetate esters, etc.) 89.27%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com