Process for preparing terephthalic acid and ethane diacid by degrading waste polyethylene glycol terephthalate plastic
A technology of polyethylene terephthalate and terephthalic acid, applied in chemical instruments and methods, bulk chemical production, carboxylate preparation, etc., can solve problems such as environmental pollution and waste of resources, and achieve high Economic benefit, low cost of degradation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
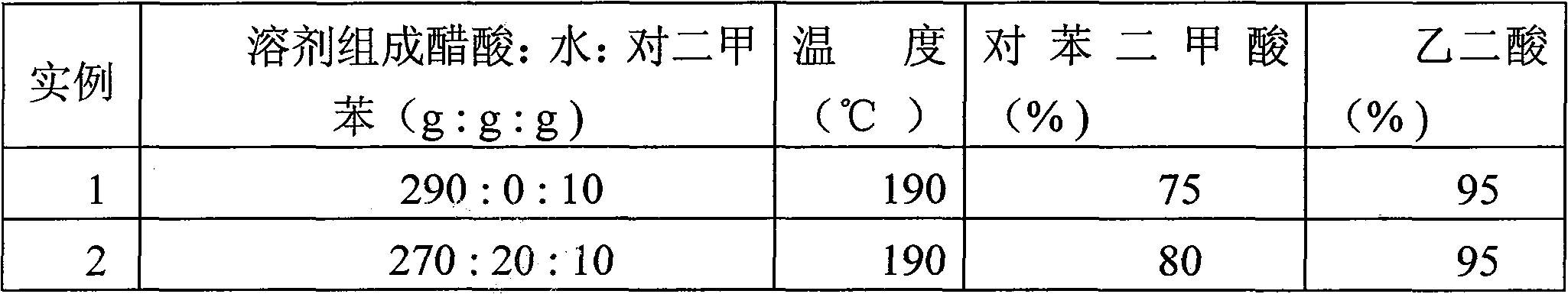
[0019] A liquid-solid reaction mixture was added to a titanium autoclave with a volume of 500 ml, nitrogen gas was introduced as a protective gas, and the temperature of the reaction mixture was raised to 190°C while stirring, and the pressure was raised to 2.0MPa. The composition of the liquid-solid reaction mixture was 290 g acetic acid, 10 g p-xylene (PX), 20 g PET solids and 3.75 g catalyst. The added catalyst consisted of: 0.76 g of cobalt acetate tetrahydrate, 0.76 g of manganese acetate tetrahydrate and 2.23 g of hydrogen bromide (47% strength aqueous solution). The degradation reaction is carried out at a temperature of 190°C and a pressure of 2.0MPa. During the reaction process, high-pressure air is continuously introduced, and the constant air flow rate is 8L / min. After 120 minutes, the reaction was terminated, and the reaction liquid sample was analyzed by capillary gas chromatography to determine the composition of its oxidative degradation small molecule product. ...
Embodiment 2
[0021] The oxidative degradation reaction of PET was carried out in the same manner as in Example 1, except that the solvent system used in Example 2 was a mixture of acetic acid and water, and the solvent composition was 270 g of acetic acid and 20 g of water. The reaction was terminated after 120 minutes of degradation, and the production amount of terephthalic acid and oxalic acid products could be measured by capillary gas chromatography. The results are shown in Table 1.
[0022] Table 1 Degradation reaction results under different solvent system composition conditions
[0023]
[0024] * The yield of terephthalic acid (%) refers to the percentage of the actual amount of terephthalic acid generated in the theoretical amount of terephthalic acid that is completely converted into terephthalic acid in PET.
[0025] Compared with Example 1, the results of Example 2 show that the acetic acid solvent system can tolerate the existence of a small amount of water molecules, an...
Embodiment 3
[0027] The oxidative degradation reaction of PET was carried out in the same manner as in Example 1, except that the degradation temperature and pressure conditions used in Example 3 were different. The degradation temperature used in Example 3 was 160°C and the pressure was 1.0MPa. The reaction was terminated after 120 minutes of degradation, and the yields of terephthalic acid and oxalic acid were determined to be 65% and 85%, respectively, by capillary gas chromatography.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com