Preparation method of (4S, 5R)- half-ester
A chiral catalyst and structural formula technology, applied in organic chemistry methods, chemical instruments and methods, asymmetric synthesis, etc., can solve the problems of expensive chiral auxiliary agents, insufficient raw material sources, harsh reaction temperature, etc. High stereoselectivity and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
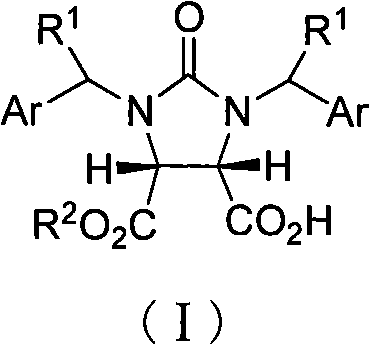
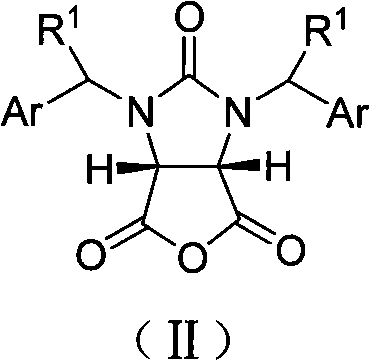
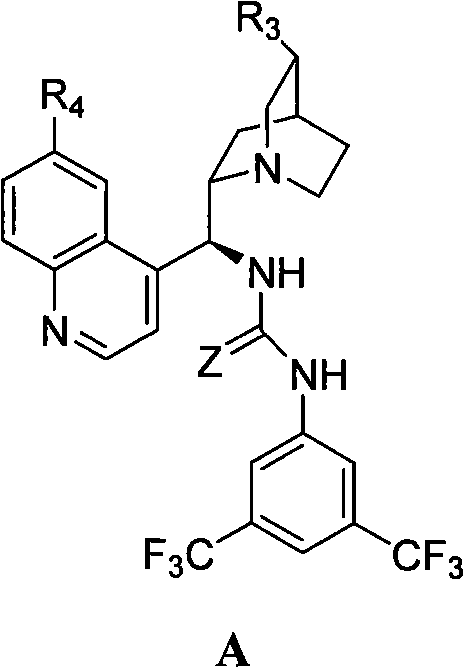
[0024] Example 1 cis-1,3-dibenzylimidazolin-2-one-2H-furo[3,4-d]imidazole-2,4,6-trione (33.6g, 0.10mol), catalyst A(R 3 =-CH=CH 2 , R 4 =-OR 5 , R 5 =CH 3 , Z=S) (65.34 g, 0.11 mol) and methyl tert-butyl ether (4 L) were placed in a dry reaction flask, anhydrous methanol (40.4 mL, 1 mol) was added dropwise at 25° C., and stirring was continued for 24 hours. After the reaction was completed, 2M hydrochloric acid (400 mL) was added to the residue, stirred for 10 min, left to stand, and the organic layer was separated and dried over anhydrous sodium sulfate. Filtration, filtrate decompression recovery solvent obtains white crystalline powder I (R 1 -H, Ar = -Ph, R 2 =-CH 3 , 36g, 98%), mp149~150℃, [α] D 22 =+2.74° (c 0.20, CHCl 3 )IR(KBr): v=2979, 2384, 2281, 1742, 1463, 1229, 1169, 767cm -1 .
[0025] 1 HNMR (CDCl 3 ): δ=3.54(s, 1H, OCH 3 ), 4.00~4.04 (m, 2H, C 6a -H,C 3a -H), 4.16~4.80 (dddd, 4H, 2×CH 2 C 6 h 5 ), 7.19~7.53 (m, 10H, 2×ArH) ppm.EI-MS: (m / z, ...
Embodiment 2
[0027] Example 2 cis-1,3-dibenzylimidazolin-2-one-2H-furo[3,4-d]imidazole-2,4,6-trione (33.6g, 0.10mol), catalyst A(R 3 =-CH=CH 2 , R 4 =-OR 5 , R 5 =CH 3, Z=S) (5.94g, 0.01mol), 1,4-dioxane (8L) were placed in a dry reaction flask, and propynyl alcohol (58.2mL, 1mol) was added dropwise at 25°C and continued to stir for 24 Hour. After the reaction was completed, 2M hydrochloric acid (40 mL) was added to the residue, stirred for 10 min, left to stand, and the organic layer was separated and dried over anhydrous sodium sulfate. Filtration, the filtrate reclaims solvent under reduced pressure, obtains white crystalline powder I (R 1 -H, Ar = -Ph, R 2 =propargyl, 37.2g, 95%), mp132.7~135.8℃, [α] D 25 =+14.3° (c 1.0, CHCl 3 ).
Embodiment 3
[0028] Example 3 cis-1,3-dibenzylimidazolin-2-one-2H-furo[3,4-d]imidazole-2,4,6-trione (33.6g, 0.10mol), catalyst A(R 3 =-CH=CH 2 , R 4 =-OR 5 , R 5 =CH 3 , Z=S) (65.34g, 0.11mol), 1,4-dioxane (4L) were placed in a dry reaction flask, and anhydrous methanol (40.4mL, 1.0mol) was added dropwise at 25°C and stirred continuously 24 hours. After the reaction was completed, 2M hydrochloric acid (400 mL) was added to the residue, stirred for 10 min, left to stand, and the organic layer was separated and dried over anhydrous sodium sulfate. Filtration, the filtrate reclaims solvent under reduced pressure, obtains white crystalline powder I (R 1 -H, Ar = -Ph, R 2 =-CH 3 , 35.2g, 96%), mp148~150℃, [α] D 22 =+2.70° (c 0.20, CHCl 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com