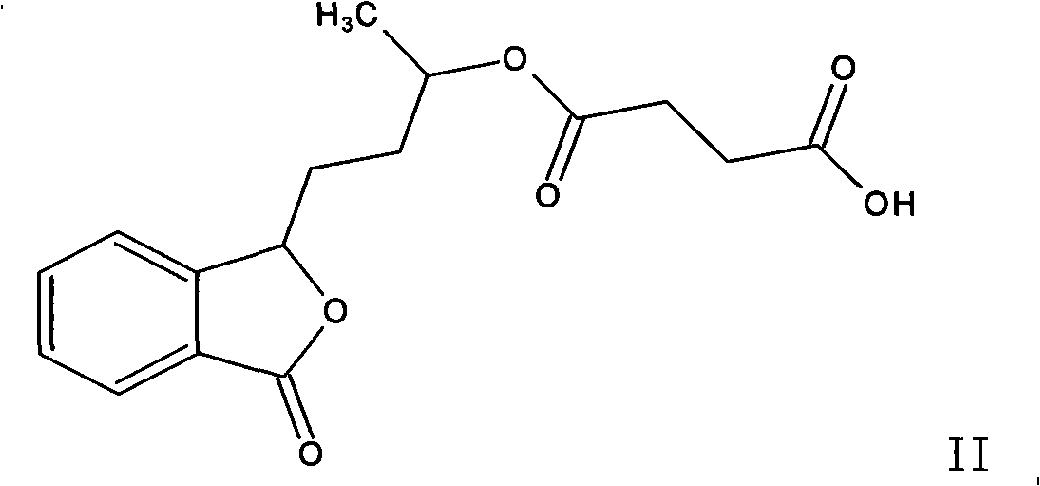
3-(3'-hydroxyl)-butyl phthalide ester, and preparation thereof and uses
A technology of butylphthalide and hydroxyl, applied in the field of 3--butylphthalide derivatives, can solve the problems of limited application, insoluble in water, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of 3'-hydroxybutylphthalide
[0032] In a 250ml three-necked round bottom flask, add 150ml of tetrahydrofuran and 2g of 60% sodium hydroxide, cool to -5℃ and stir, then add 13.4g (0.1mol) phthalide, keep the temperature at -5℃-0℃, and stir React for 30 minutes. The mixture was a light green solution. At this time, drop in a 50ml tetrahydrofuran solution containing 16.4g (0.1mol) of 3-hydroxybromo-n-butane. Control the dropping rate to keep the temperature at 0°C. Keep the reaction after the dropping, TLC The thin layer makes the raw material point of phthalide disappear. At this time, 8ml of distilled water was added dropwise, the mixture was added to 300ml of ethyl acetate, washed twice with 50ml of saturated sodium hydride solution, and then washed with 50ml of water twice, dried with anhydrous sodium sulfate for 2 hours, filtered, and the filtrate was concentrated to dryness to obtain a nearly white color. Crystalline solid. It was vacuum dried in ...
Embodiment 2
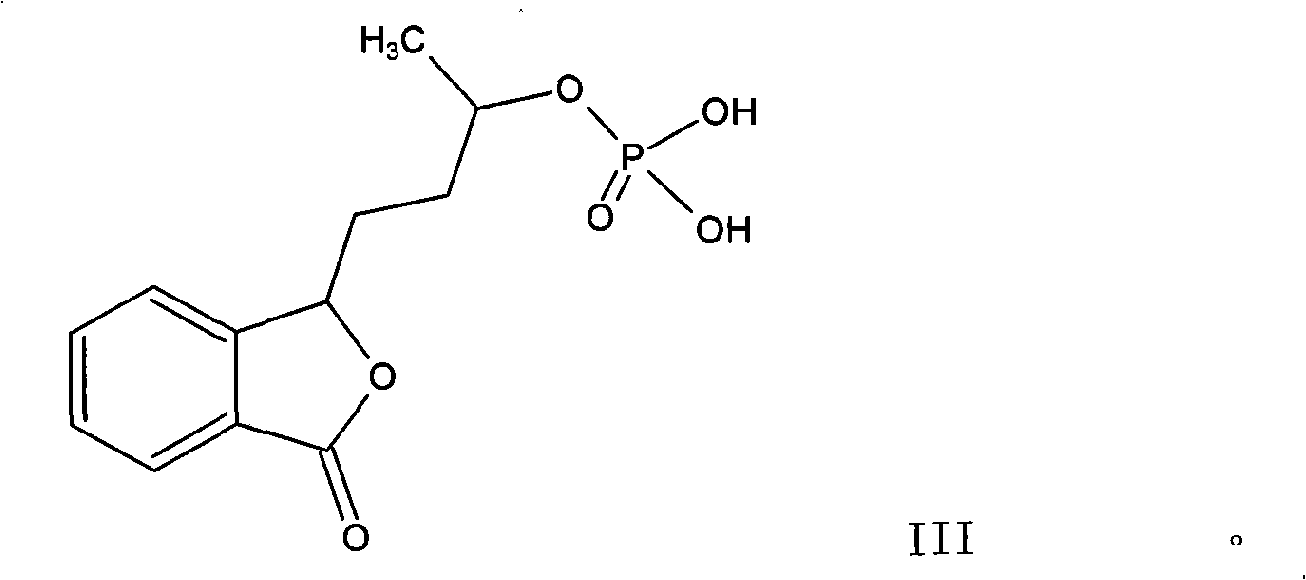
[0035] Example 2: Preparation of 3-(3'-phosphoric acid) butylphthalide and its sodium salt
[0036] In a 250ml three-necked flask, add 150ml of N,N-dimethylformamide and 20ml of pyridine, then cool to -10°C and drop 25ml of phosphorus oxychloride. After the drop is stirred for 20 minutes, add a total of 20.7g3 to it. '-Hydroxybutylphthalide, stir and react for 3 hours, after the thin-layer identification reaction is completed, pour the mixture into water, adjust the pH to 2-3 with dilute hydrochloric acid, extract twice with 200ml ethyl acetate, and wash the extract with water To neutral. Dry with anhydrous sodium sulfate and concentrate to dryness to obtain 3-(3'-phosphoric acid) butylphthalide. 3-(3'-phosphoric acid) butylphthalide was dissolved in 95% ethanol, 10.6g of sodium carbonate was added, and the reaction was stirred at 30°C for 1 hour. Then, it was added to 100ml of acetone to cool, and a white crystalline solid was precipitated, which was dried under vacuum. Obtained ...
Embodiment 3
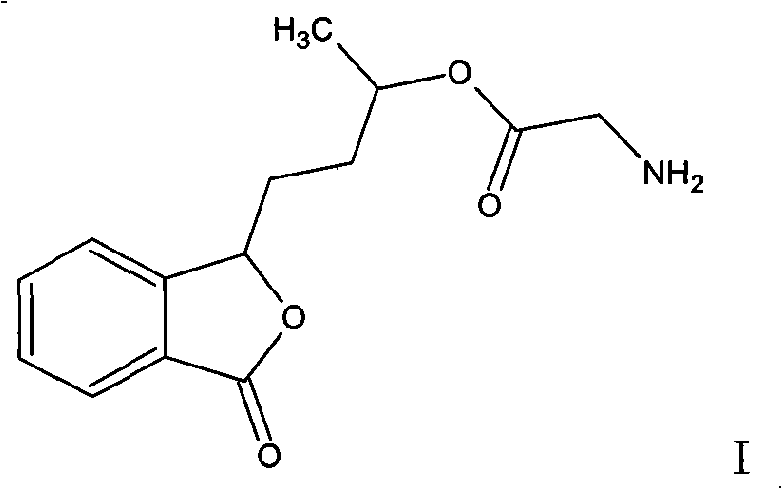
[0040]Example 3: Preparation of 3-(3'-glycinate)butylphthalide and 3-(3'-glycinate)butylphthalide hydrochloride
[0041] In a 500ml three-necked flask, add 20.7g of 3'-hydroxybutylphthalide, 150ml of ethyl acetate, cool to 0°C, add 5ml of anhydrous pyridine and 1g of DMAP, add 6g of glycine while stirring, keep the temperature to 0-5°C, After the reaction is complete, pour the mixture into 50ml ice water, adjust the pH value to neutral with 0.1N hydrochloric acid, stand for separation, save the organic layer, extract the aqueous layer twice with 50ml ethyl acetate, combine the organic phases, wash twice with 50ml water Then, it was dried with anhydrous magnesium sulfate, filtered, and concentrated to dryness to obtain 16.7 g of 3-(3'-glycinate)butylphthalide with a yield of 58%.
[0042]
[0043] 1H-NMR (400MHz, CDCl3δppm): 1.14~1.17(3H, m, H4'), 1.45~1.70(1H, m, H2'), 1.86~2.24(1H, m, H1'), 3.64(2H, m ,H6')3.74--3.82(1H,m,H3'),5.46-5.50(1H,m,H2),7.42-7.44(1H,m,Ar-H6),7.48(1H,m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com