Method for preparing tetrahydrothiazole diketone derivatives
A technology for thiazolidinedione and derivatives, which is applied in the field of preparation of thiazolidinedione derivatives, can solve problems such as high reaction temperature, low production efficiency, and environmental pollution, and achieve simple process operation, reduced production cost, and product separation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
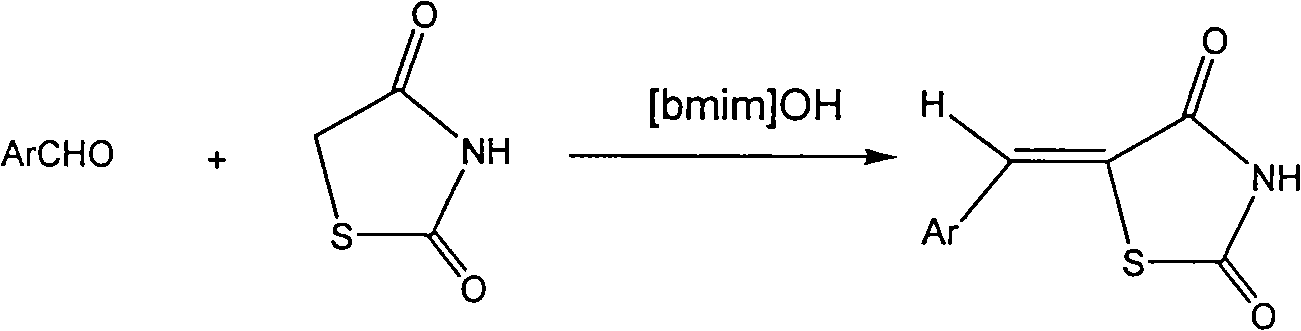
[0025] Preparation of [Bmim]OH Ionic Liquid
[0026] 1. Preparation of [Bmim]Br
[0027] Heat 0.5mol N-methylimidazole to 70°C in advance, and slowly add 0.55mol bromobutane dropwise under strong stirring. After the dropwise addition, continue to raise the temperature to 90°C, and keep the reaction for 5h.
[0028] To the end of the reaction, the reaction solution was poured out, washed with ethyl acetate for 3 to 4 times, and the washed reaction solution was rotary evaporated to remove residual ethyl acetate to obtain a white solid [Bmim]Br.
[0029] 2. Preparation of [Bmim]OH from [Bmim]Br
[0030] Take 0.3mol [Bmim]Br and 0.3mol potassium hydroxide, add 100ml of anhydrous dichloromethane as solvent, stir at room temperature for 10h, and end the reaction. The precipitate was removed by filtration, the filtrate was rotary evaporated to remove dichloromethane, and then washed with diethyl ether for 3 to 4 times to obtain the basic ionic liquid [Bmim]OH.
Embodiment 1
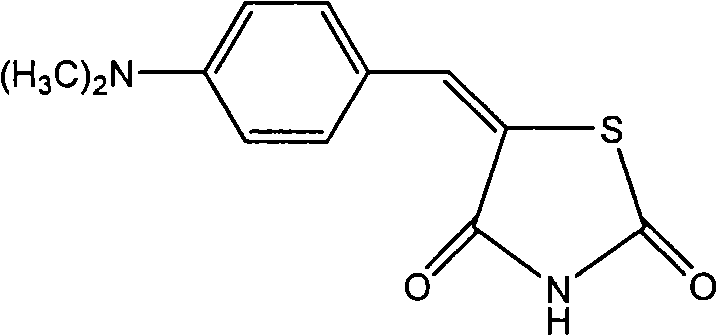
[0032] Preparation of 5-(4-dimethylamino)-benzylidene-2,4-thiazolidinedione
[0033]
[0034] Add 4mmol of 2,4-thiazolidinedione and 4mmol of p-dimethylaminobenzaldehyde into a 25ml round bottom flask, add 20ml of ionic liquid [Bmim]OH, stir at 100°C, and track the reaction process by thin layer chromatography (TLC). After about 10 hours, the reaction was completed. Add 2ml of distilled water to the reaction solution, continue to stir, stop stirring after 5min, filter, wash the residue once with distilled water, and collect the crude product after drying. The crude product was recrystallized with absolute ethanol to obtain the pure product 5-(4-dimethylamino)benzylidene-2,4-thiazolidinedione compound with a yield of 92%, m.p: 296-297°C, purity 99%.
[0035] Recovery of ionic liquid: Distill the filtered filtrate at 75-80°C to remove moisture under reduced pressure to obtain pure ionic liquid for the next round of preparation.
[0036] Characterization of this compound: 1...
Embodiment 2
[0038] Preparation of 5-(4-methoxy)-benzylidene-2,4-thiazolidinedione
[0039]
[0040] Add 0.8mmol of 2,4-thiazolidinedione and 4mmol of p-methoxybenzaldehyde into a 25ml round bottom flask, add 8ml of ionic liquid [Bmim]OH, stir at 100°C, and track the reaction process by thin layer chromatography (TLC) After about 10 hours, the reaction was completed. Add 2ml of distilled water to the reaction solution, continue to stir, stop stirring after 5min, filter, wash the residue once with distilled water, and collect the crude product after drying. The crude product was recrystallized with absolute ethanol to obtain the pure product 5-(4-methoxy)benzylidene-2,4-thiazolidinedione compound with a yield of 91%, m.p: 218-219°C, purity 99%.
[0041] Recovery of ionic liquid: Distill the filtered filtrate at 75-80°C to remove moisture under reduced pressure to obtain pure ionic liquid for the next round of preparation.
[0042] Characterization of this compound: 1 H NMR (400MHz, DM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com