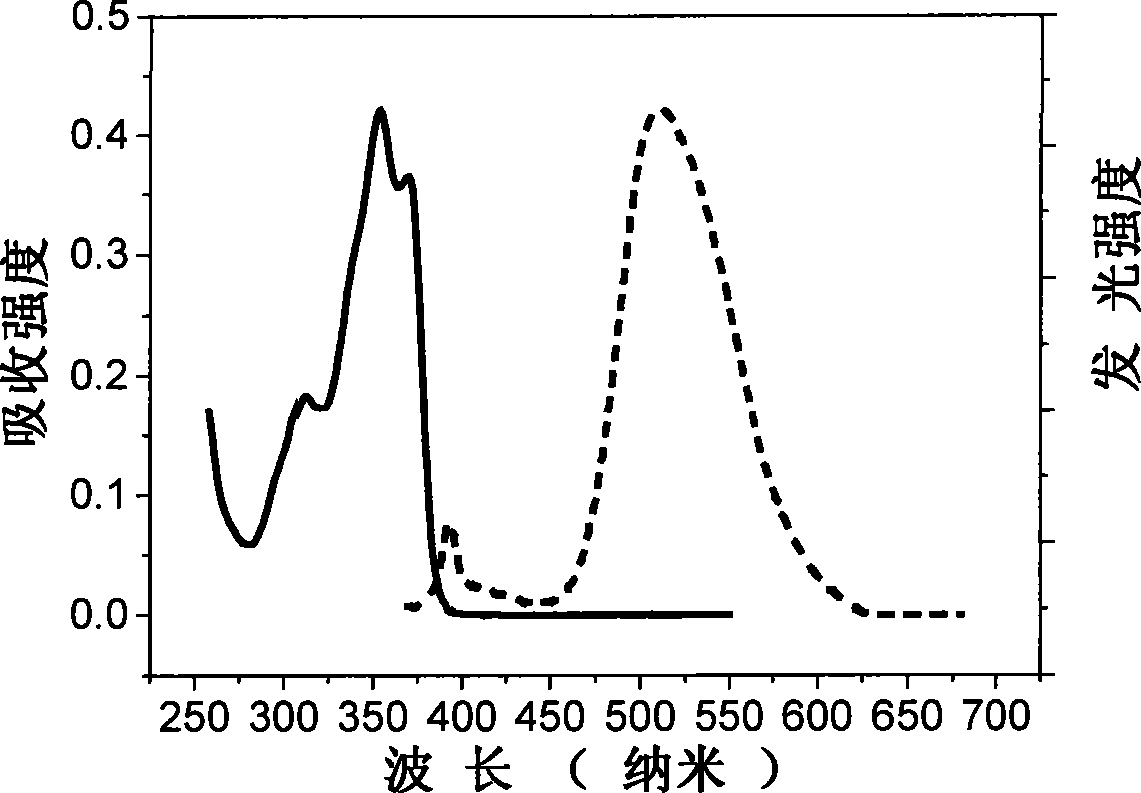
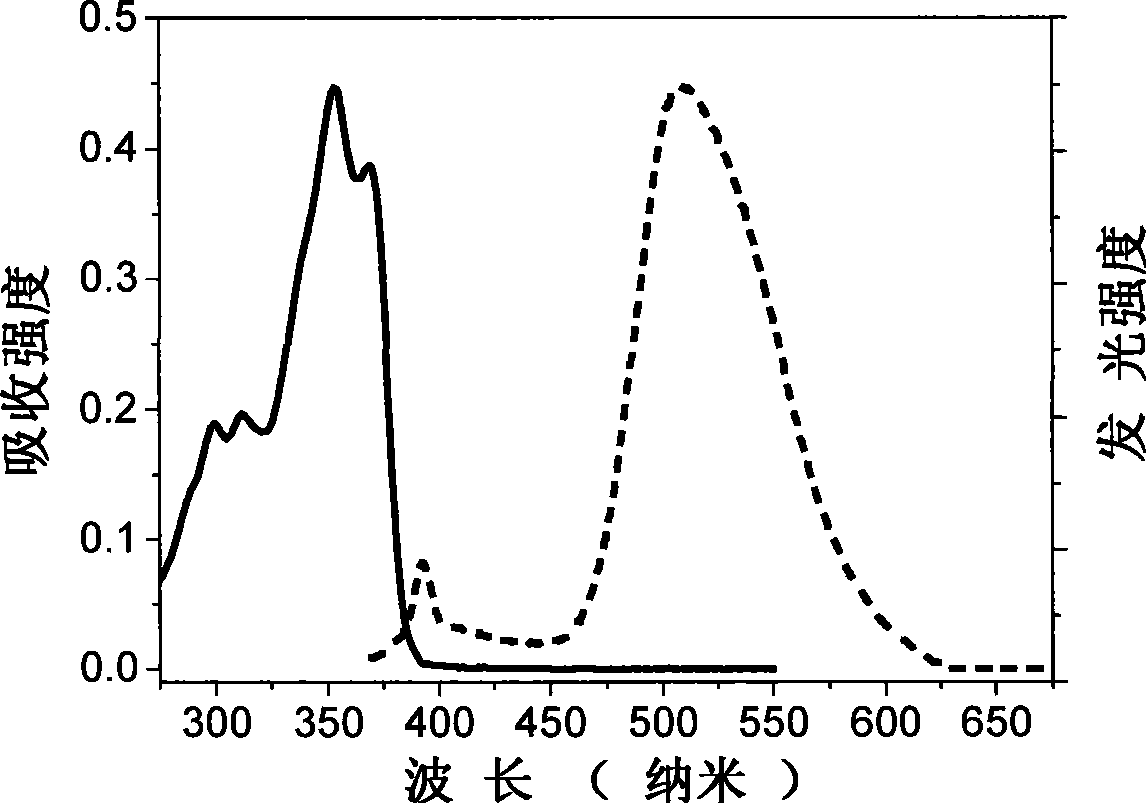
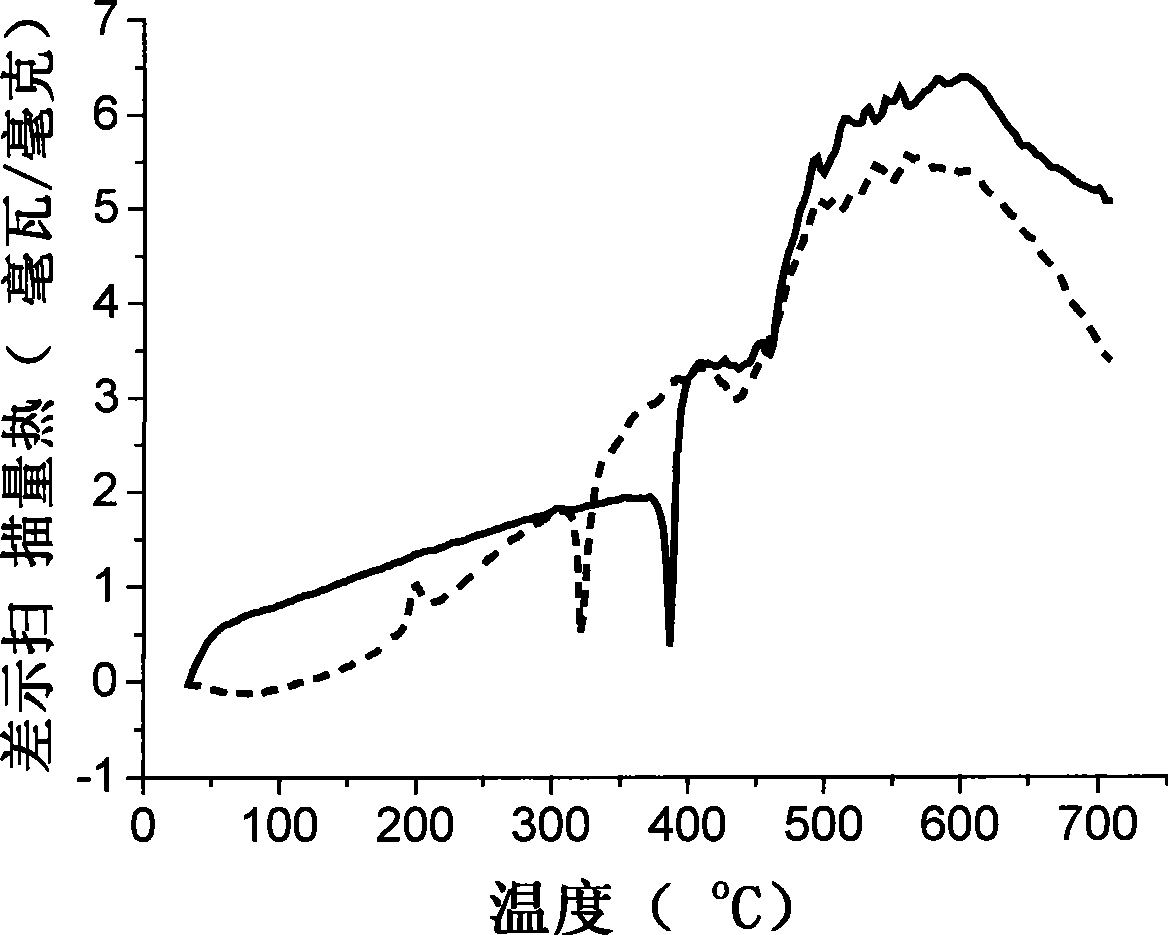
Acidamide compound based on 2-(2'-hydroxyphenyl) benzoxazole, preparation and use thereof
A technology for amide compounds and amine compounds, applied in the field of preparing organic light-emitting materials, can solve the problems of inability to achieve acylation, inability to easily prepare amide compounds, strong corrosiveness, etc., and achieve good thermal stability, separation and purification of products Easy operation, high decomposition temperature effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
[0035] Examples 1 to 3 are preparation examples of amine compounds of 2-(2'-hydroxyphenyl)benzoxazole (HBX).
Embodiment 1
[0037] Preparation of 2-(4'-amino-2'-hydroxyphenyl)benzothiazole, according to literature 1 (Ermanno, B.; Piero, S.; Mario, M.; Macro, P.J.Heterocyclic.Chem.1983,20, 1517) method is synthesized, and its molecular structural formula is:
[0038]
[0039] Add 12.2g of o-aminobenzenethiol (0.1mol) and 15.1g of 4-aminosalicylic acid (0.1mol) into 100ml of polyphosphoric acid (PPA), stir at 160°C for 6 hours under nitrogen protection, and react After the liquid is cooled, dilute it with a large amount of ice water, and then use NaOH solution and NaHCO 3 The solution was adjusted to neutral, and a large amount of precipitates were precipitated, which were filtered to obtain the pale yellow primary product (86%). Then, the product was purified by quickly passing through a silica gel column with an eluent of ethyl acetate:cyclohexane=3:1 (volume ratio).
Embodiment 2
[0041] Preparation of 2-(4'-amino-2'-hydroxyl phenyl) benzodummy azole is synthesized according to the method of document 1, and its molecular structural formula is:
[0042]
[0043] The raw material o-aminobenzenethiol is changed to o-aminophenol and other steps are the same as in Example 1,
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com