Acidum folicum effervescent tablet and preparation thereof
A technology of effervescent tablets and folic acid, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problems of hindering the production of effervescent folic acid tablets, increased perinatal deaths, and low birth rates. Weight gain and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
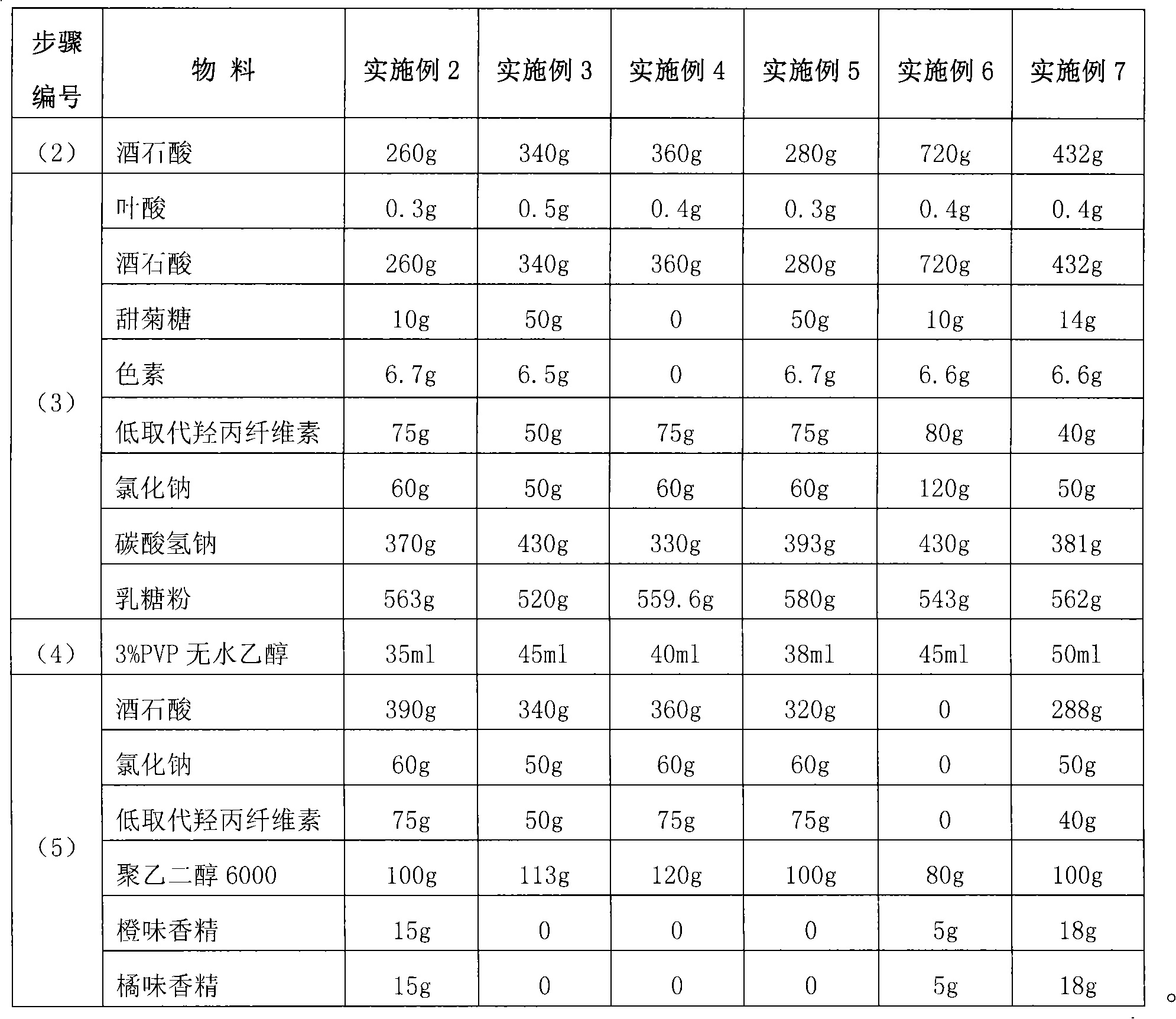
[0065] [Example 1] Folic acid effervescent tablet
[0066] prescription:
[0067] The dosage per 1000 tablets is
[0068] Folic acid 0.4g
[0069] Stevia 35g
[0070] Pigment 6.6g
[0071] Tartaric acid 685g
[0072] Sodium bicarbonate 400g
[0074] Macrogol 6000 100g
[0075] Lactose 553g
[0076] Low-substituted hydroxypropyl cellulose 80g
[0077] Orange Flavor 20g
[0078] Orange flavor 20g
[0079] Preparation Process:
[0080] (1) Material drying
[0081] Dry tartaric acid, sodium bicarbonate, lactose, sodium chloride, and low-substituted hydroxypropyl cellulose at below 50°C, and dry polyethylene glycol 6000 at below 30°C, so that the water content of each material is ≤1.5%;
[0082] (2) Crushing and screening
[0083] Sodium bicarbonate, 342.5g of tartaric acid, polyethylene glycol 6000, lactose, and low-substituted hydroxypropyl cellulose were pulverized respectively, and passed through an 80-mesh sieve; the particle size of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com