Anion-exchange displacement chromatography process and anionic organic compounds for use as displacer compounds in anion-exchange displacement chromatography
A displacing agent and compound technology, which is applied in the field of compositions containing polyvalent organic anion salts, can solve the problems of reducing the advantages of displacement chromatography, difficult synthesis and purification of compounds, and inability to obtain commercially at a cost, so as to achieve simple and effective synthesis and purification The effect of reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
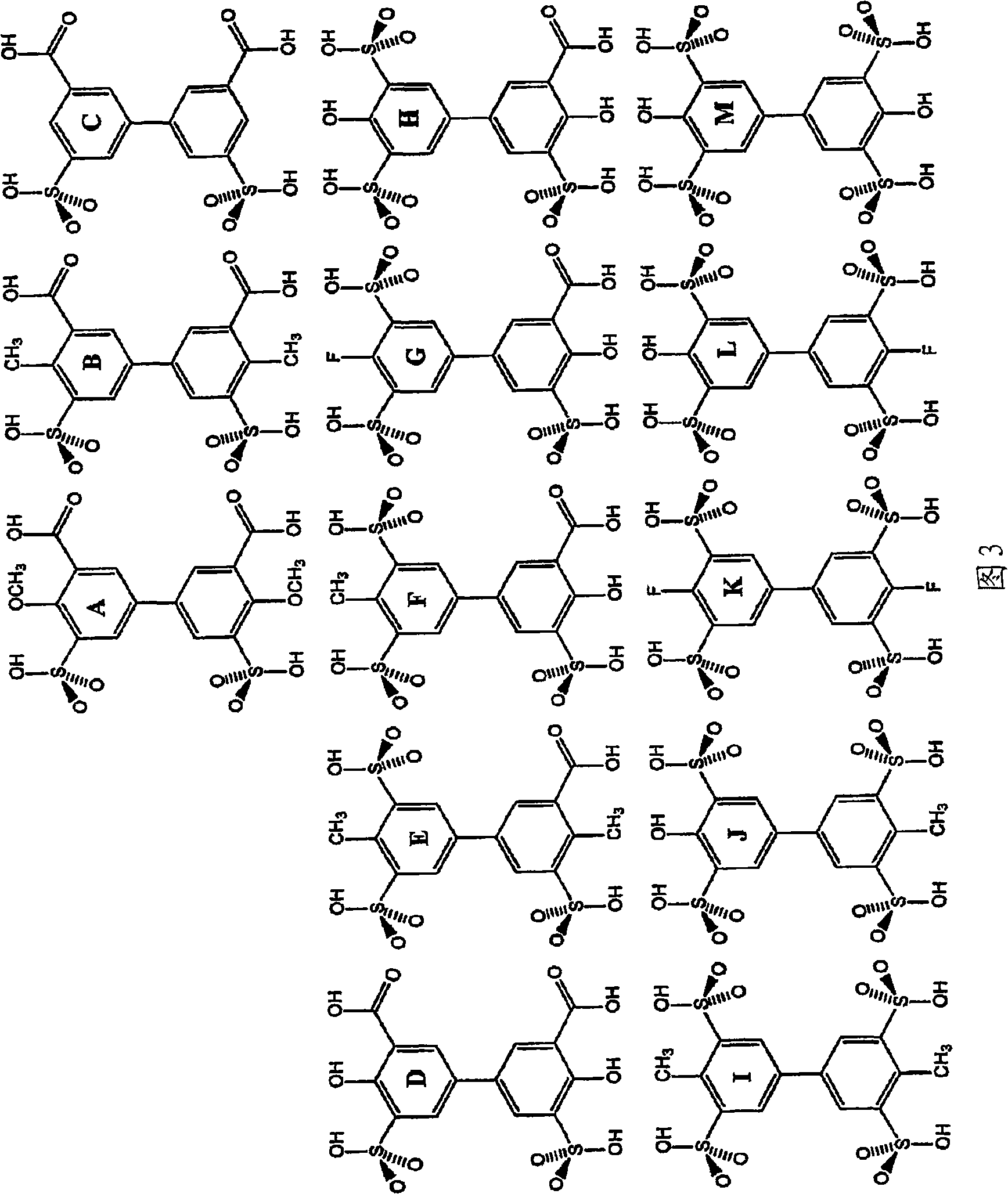
Embodiment 1-AD-1
[0154] The synthesis of embodiment 1-AD-1
[0155]Add 500 mL of concentrated sulfuric acid and a magnetic stir bar to a 1000 mL 3-neck round bottom flask equipped with a thermocouple coated with tetrafluoroethylene. The flask was purged with dry nitrogen while 35 g of tetraphenylethylene was added to the powder addition funnel. A powder addition funnel was placed in place in the round bottom flask and the flask was sealed under an atmosphere of dry nitrogen at a pressure slightly above ambient. The sulfuric acid was heated to about 115°C with stirring, and the tetraphenylethylene was added to the sulfuric acid in small portions over about 1 hour. Each portion of tetraphenylethylene should be added after the preceding portion has completely dissolved in the liquid reaction mixture. When the addition was complete, the reaction mixture was maintained at 115°C in the dark for about 1 hour and then cooled to room temperature. The cooled reaction mixture was transferred to an add...
Embodiment 2-AD-2
[0156] The synthesis of embodiment 2-AD-2
[0157] The synthesis of AD-2 can be carried out through the following two steps.
[0158] step 1: Synthesis of 2-chloro-4,6-bis(3-carboxy-4-hydroxy-5-sulfoanilino)-1,3,5-triazine disodium salt (molecular weight=621.85)
[0159] 23g of distilled water and 56g of ice (from the distilled water) were placed in the flask and stirred magnetically. The pH and temperature of subsequent reactions were monitored with glass pH probes and temperature probes. 100 mg of Triton-X100 detergent was added to the stirred mixture, followed by 1.90 g of cyanuric chloride (Aldrich) (10.3 mmol, molecular weight = 184.4) in one portion. For the first part of the reaction, the temperature can be maintained at 0-5 °C by external cooling, if necessary, by adding a small amount of solid LiOH.H 2 O keeps the pH at 3.5-4.0. After adding in portions over two hours, a small amount of solid 5-amino-3-sulfosalicylic acid (4.8 g, 20.6 mmol, Aldrich) was added to...
Embodiment 3
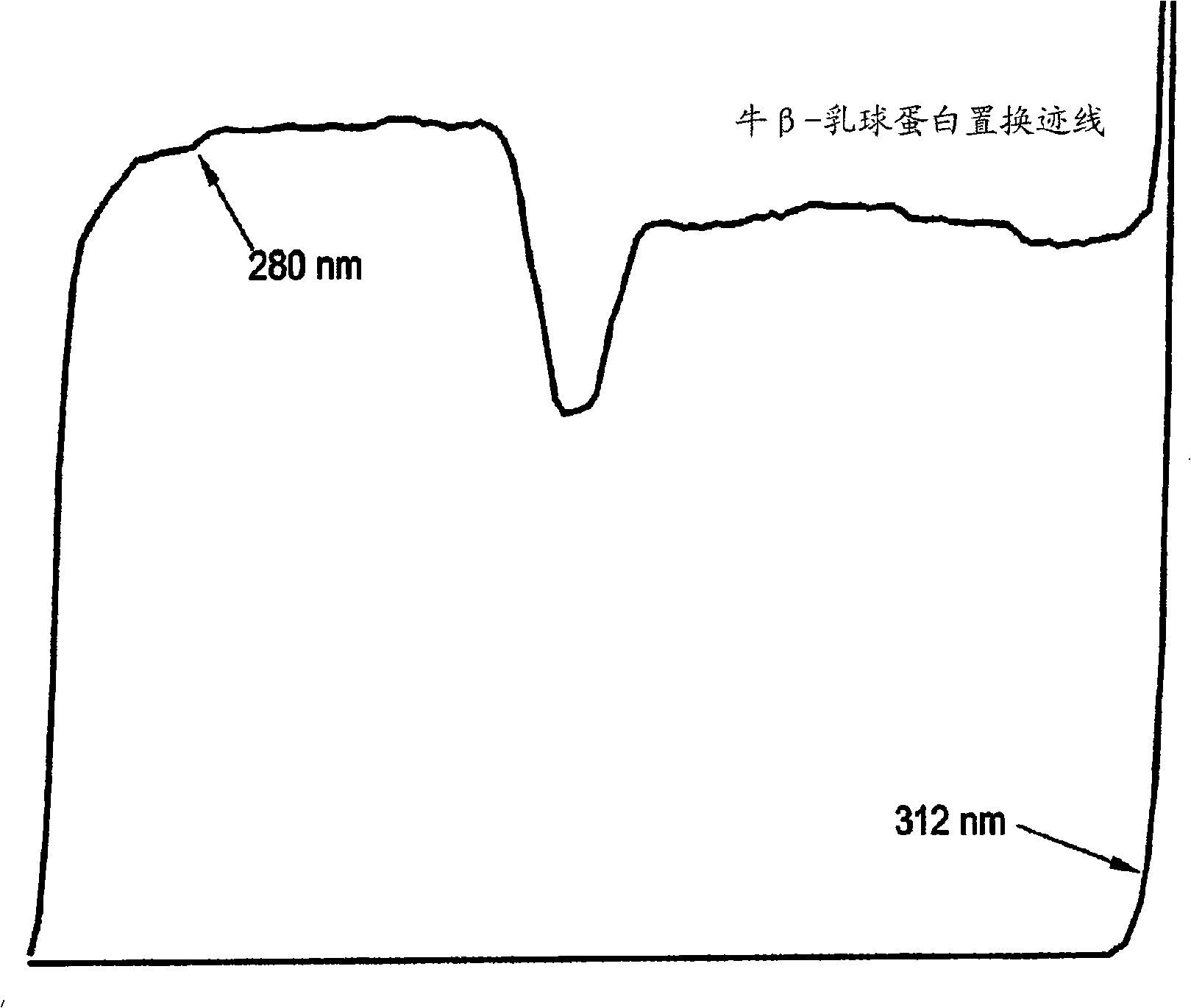
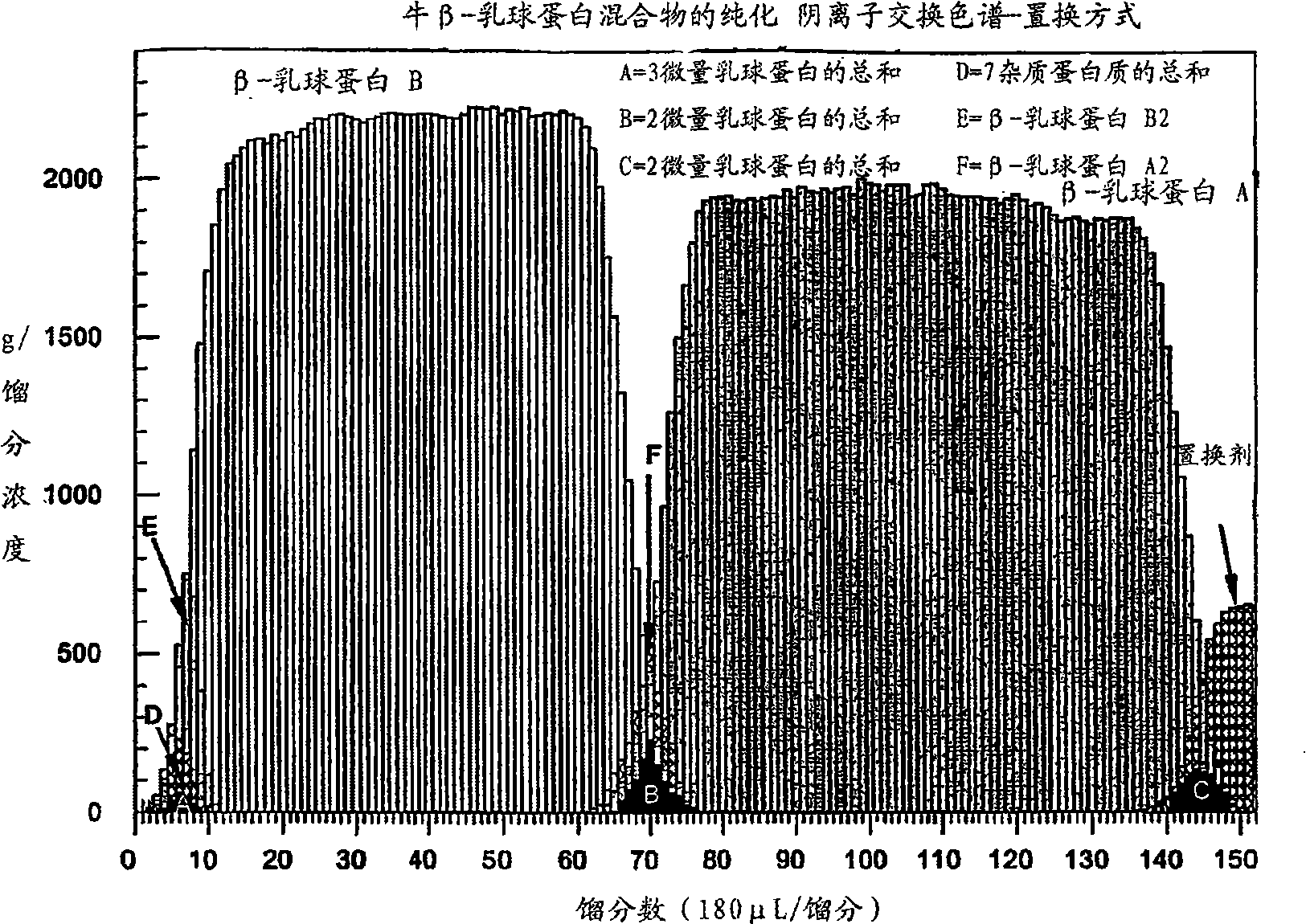
[0162] Example 3 - Displacement Chromatography of Protein Mixtures
[0163] Penetration test. A 5 mM solution of bovine β-lactoglobulin A in loading buffer and bovine β-lactoglobulin B in loading buffer was used. Breakthrough experiments were performed at different flow rates between 0.1-0.5 mL / min. The loading buffer solution was made of 25 mM BHEP (1,4-bis(hydroxyethyl)piperazine) and adjusted to pH=7.7 with hydrochloric acid. Reproducible results were obtained at a flow rate of 0.18mL / min. The column saturation capacity for β-lactoglobulin B was 459 mg (108 mg / mL) and that for β-lactoglobulin A was 463 mg (109.2 mg / mL). In these experiments, unpurified lactoglobulin (Sigma) was taken directly from the bottle and used.
[0164] Column preparation. Clean and regenerate the column (Tosoh SuperQ-5PW, 6.0 x 150mm) with Method A (below), then store as chloride in NaCl buffer solution of 2.0M NaCl + 25Mm BHEP , adjust pH=7.7 with hydrochloric acid. The output of the colum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com