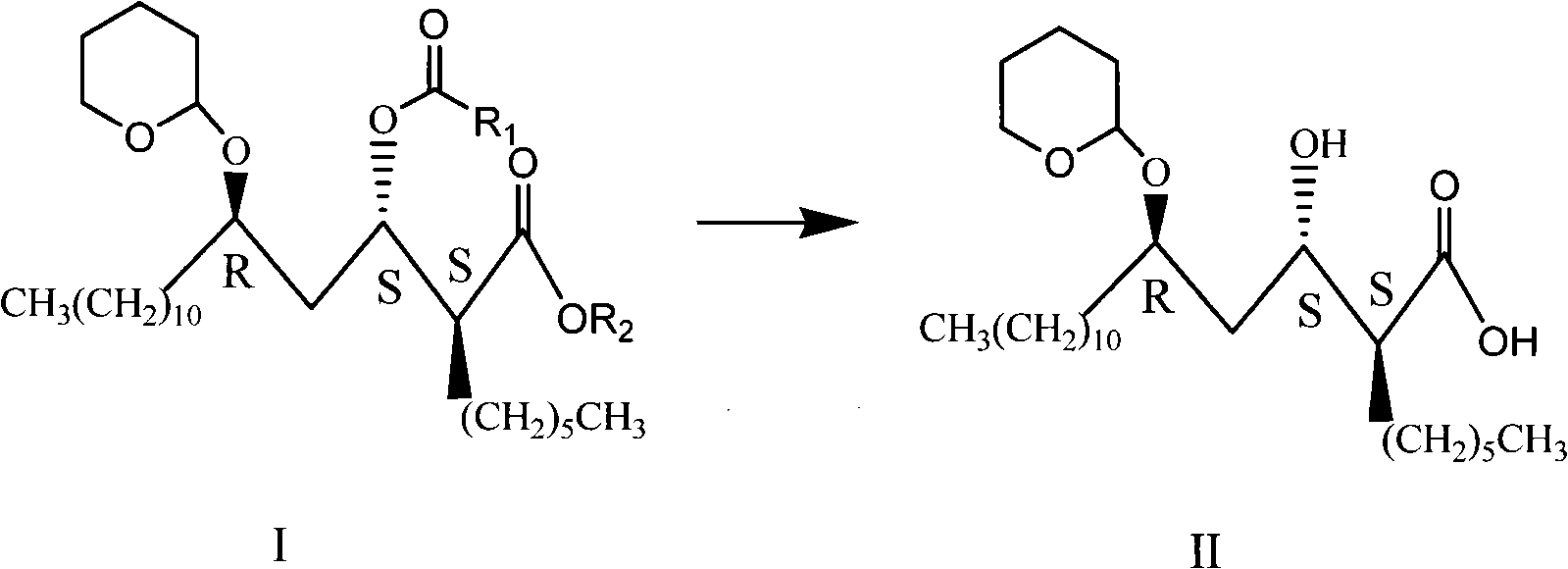
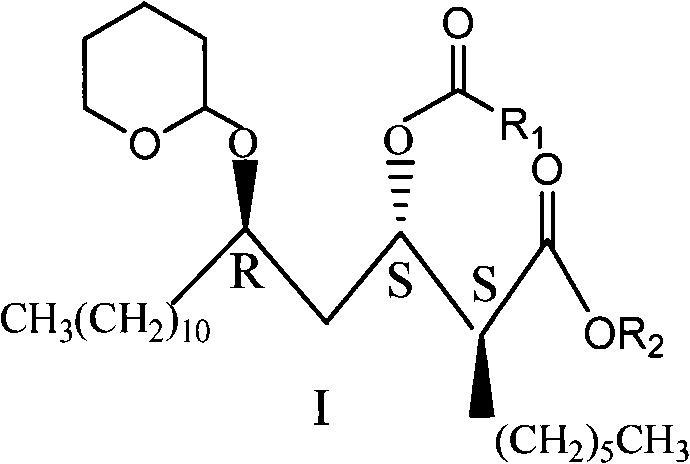
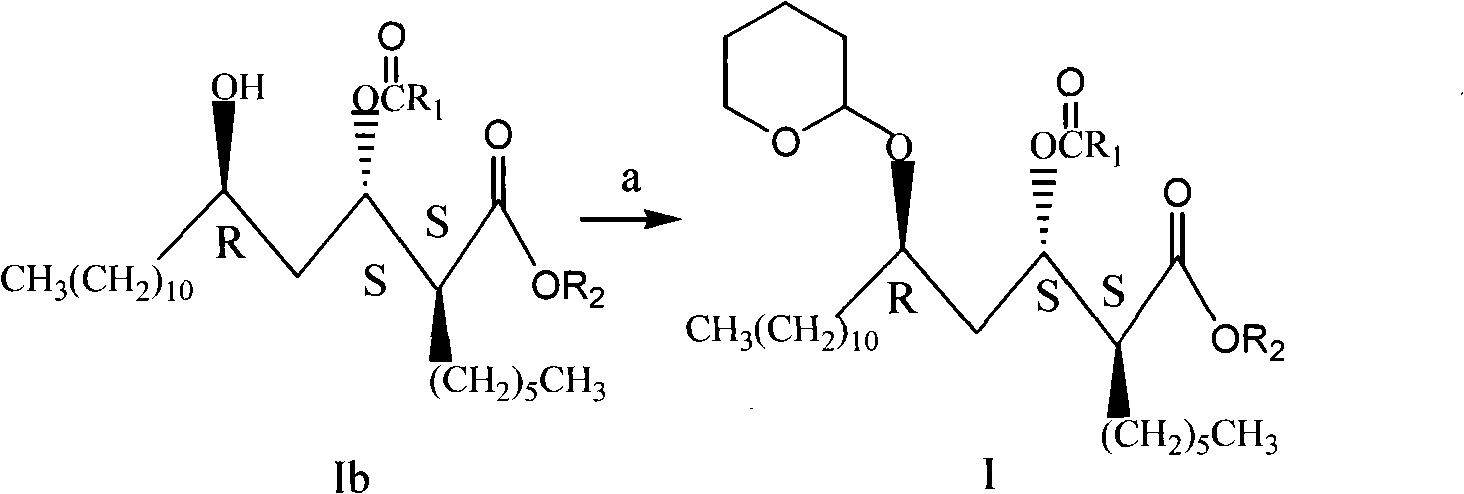
Novel method for synthesizing orlistat, intermediate compound and preparation thereof
An orlistat and compound technology, which is applied in the field of synthesis of lipid-lowering drug compounds, can solve the problem that it cannot be fully used for industrialization and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of (2S, 3S, 5R)-3-hydroxy-2-hexyl-5-undecyl-valerolactone (1)
[0049] In the dry reaction bottle, add 5-(R)-3-keto-2-hexyl-5-undecyl-valerolactone (refer to Martin Karpf, et al., US5399720 preparation) 141g (0.4mol) and 3.5 L of ethyl acetate was stirred evenly, 52 g of Raney nickel was added, hydrogen gas was introduced at normal pressure, the temperature was controlled at about 30°C, and the reaction was stirred for 24 hours. TLC detection (developing agent: n-hexane: ethyl acetate = 10: 3, silica gel is GF254, observed under iodine smoked color development, the R of raw material f = 0.6). After the reaction is complete, filter and distill at normal pressure until the remaining 250 mL remains. Set aside to crystallize at about -10°C overnight, filter, collect the filter cake, and dry at 60°C for 10 hours under normal pressure to obtain 115 g of white solid. Yield: 81%, m.p.102.2~103.6℃, [α] 20 D +47° (C=1, chloroform). Anal.C 22 h 42 o 3 : C, 74...
Embodiment 2
[0052] Preparation of Compound Ia
[0053] a) Preparation of (2S, 3S, 5R)-2-hexyl-3-benzoyloxy-5-undecyl-valerolactone (10)
[0054] In a dry reaction flask, add compound (1) 48g (0.136mol) and dichloromethane 480mL, under stirring, add 4-dimethylaminopyridine 4.2g, benzoyl chloride 16.8g (0.12mol) and triethylamine 14.2 g (0.14mol), at room temperature, stirring and reacting for 24 hours, TLC detection (developing solvent: n-hexane: ethyl acetate=10:3, silica gel is GF254, observed under iodine smoked color development, the R of the raw material f =0.53), after the reaction is complete, use saturated sodium carbonate solution pH8~9, separate the organic layer, wash the organic layer with water, dry the organic layer with anhydrous sodium sulfate for 3 hours, concentrate the filtrate under normal pressure and evaporate to dryness under reduced pressure, add Methanol 100mL, refrigerated and crystallized overnight, filtered, and the filter cake was dried under normal pressure a...
Embodiment 3
[0058] Preparation of compound Ib
[0059] a) Preparation of (2S, 3S, 5R)-3-benzoyloxy-2-hexyl-5-hydroxyhexadecanoic acid methyl ester (11)
[0060] Add 70 g (0.153 mol) of compound (10) and 350 mL of methanol into the reaction flask, stir evenly, add 0.7 mL of concentrated sulfuric acid, and stir for reaction at room temperature for 24 hours. TLC detection (developing agent: n-hexane: ethyl acetate = 10: 3, silica gel is GF254, observed under iodine smoked color development, the R of raw material f =0.77), after the completion of the reaction, add triethylamine to adjust the pH to about 9, distill under reduced pressure, after the distillation is completed, add 500 mL of n-hexane and 500 mL of water, stir for 10 minutes, separate the organic layer, extract the water layer twice with n-hexane, and combine , washed twice with water, the organic layer was dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 68 g of li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com