Catalyst for producing acroleic acid by lactic acid dewatering and reaction technique
A catalyst and acrylic acid technology, applied in molecular sieve catalysts, physical/chemical process catalysts, carboxylate preparation, etc., can solve the problems of low conversion rate and yield of lactate reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
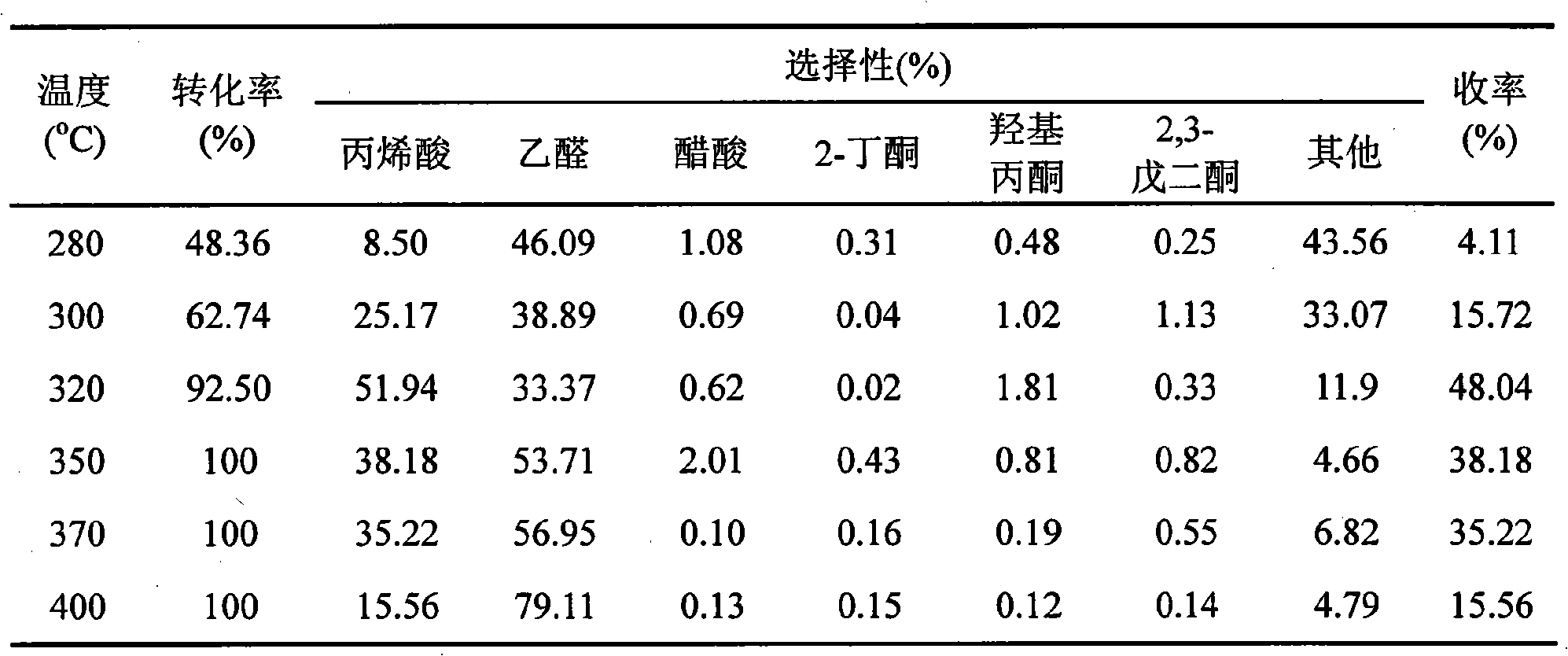
[0010] Weigh 4g of HZSM-5 (Si / Al=30) powder, add it into 84.9ml KCl solution with a concentration of 0.2mol / L, stir evenly, and let it stand at room temperature for 24 hours. Then remove the clear liquid part, and dry the remaining emulsion part overnight at 100 °C, and then bake it in a muffle furnace at 450 °C for 4 hours to obtain K 2 K-HZSM-5 catalyst with calculated O content of 20 wt%. After the catalyst was tableted and granulated, the 30-mesh particles were screened for the reaction of lactic acid dehydration to acrylic acid.
[0011] The middle part of the quartz tube fixed-bed reactor with a length of 30 cm is filled with catalyst, and the height of the catalyst bed is 3 cm. The space above the catalyst bed in the reaction tube is filled with 30-mesh quartz sand. The carrier gas nitrogen with a flow rate of 20ml / min was introduced to keep the pressure at 0.4MPa. A lactic acid solution with a concentration of 30wt% was introduced into the reaction tube with a micro...
Embodiment 2
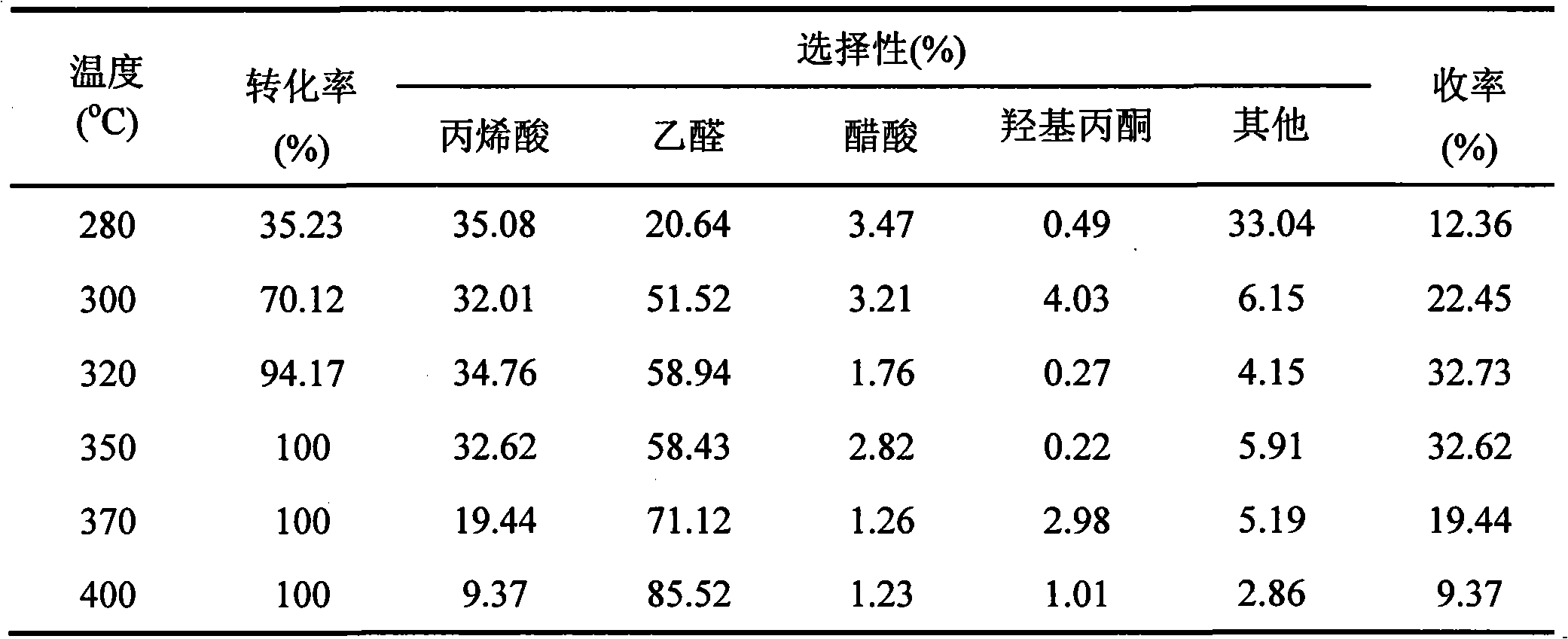
[0016] Weigh 4g of HZSM-5 (Si / Al=30) powder and add it into 267.7ml of lithium nitrate solution with a concentration of 0.2mol / L, stir evenly, and let it stand at room temperature for 24 hours. Then the clear liquid part was removed, and the remaining emulsion part was dried overnight at 100 °C, and then baked in a muffle furnace at 450 °C for 4 hours to obtain Li 2 Li-HZSM-5 catalyst with a calculated O content of 20 wt%. After the catalyst was tableted and granulated, the 30-mesh particles were screened for the reaction of lactic acid dehydration to acrylic acid. The reaction conditions are the same as in Example 1, and the results are shown in Table 2.
[0017] Table 2 The reaction results of lactic acid dehydration to acrylic acid at different reaction temperatures on Li-HZSM-5 catalyst
[0018]
[0019] *Other products are mainly 2-butyraldehyde, acetone, hydroxyacetone and polylactic acid.
Embodiment 3
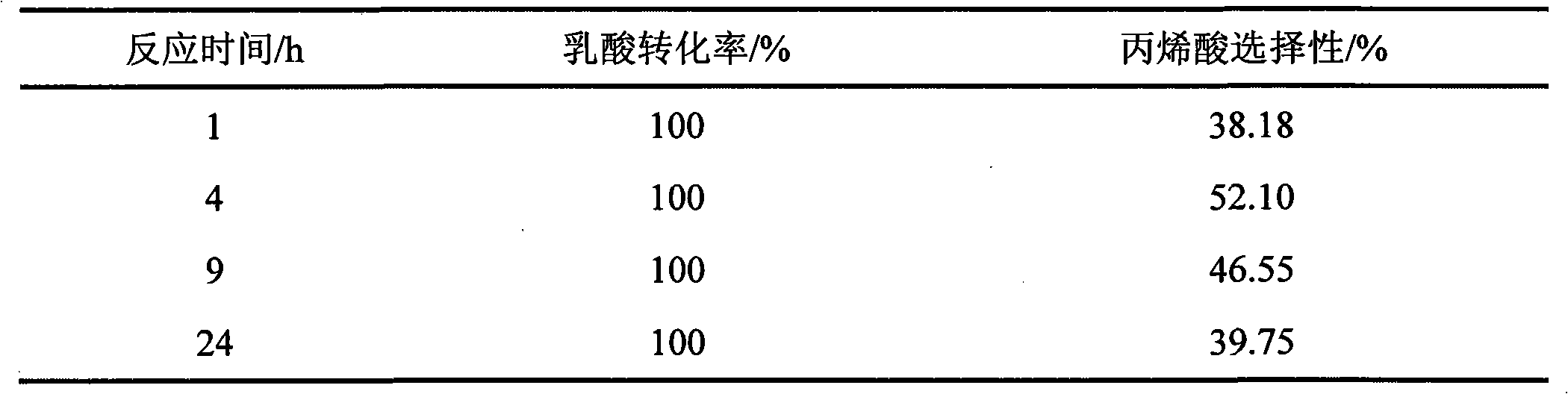
[0021] Prepare K-HZSM-5 catalyst according to embodiment 1. Lactic acid catalytic dehydration reaction conditions: except that the reaction temperature was fixed at 350° C. and different reaction times were used, the others were the same as in Example 1. The reaction results are shown in Table 3.
[0022] Table 3 The reaction results of lactic acid dehydration to acrylic acid with different reaction times on K-HZSM-5 catalyst
[0023]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com