Continuous gas phase reaction method of isoprene-3-methyl butan-2-alkenyl ether
A technology of isoprenyl and alkenal diisoprenyl acetal is applied in the field of preparation of isoprenyl-3-methylbut-2-enyl ether, and can solve the requirement of phosphoric acid concentration control High, poor stability, difficulty and other problems, to reduce the operation of solvent recovery, improve the yield, and achieve the effect of less destructive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of 3-methylbut-2-enal di-isoprenyl acetal
[0033] In a 1000ml three-neck flask equipped with mechanical stirring, a 1.0m rectification tower (20 theoretical plates of the rectification tower), a water separator, and a thermometer, 100ml of cyclohexane was put in, and 3-methyl-2-butyl Alkenal 168g (2mol), 3-methyl-2-butenol 361g (4.2mol), adipic acid 5g, heat up and reflux, control the reaction temperature at 80-85°C, and separate the generated water in the water separator After 10 hours of reaction, the reaction was terminated, unreacted raw materials were recovered by distillation under reduced pressure, and then rectified under reduced pressure to obtain 296 g of 3-methylbut-2-enal di-prenyl acetal, with a content of 97%.
Embodiment 2
[0034] Embodiment 2 Preparation of Elimination Reaction Catalyst
[0035] 2 parts of calcium phosphate, 3 parts of diammonium hydrogen phosphate, 1 part of potassium dihydrogen phosphate, and 3 parts of graphite (parts by weight) are mixed evenly, pressed into tablets, baked at 250-280°C for 3 hours in an isolated air, crushed, sieved, and selected The 30-80 mesh sieve is used as the catalyst for elimination.
Embodiment 3-5
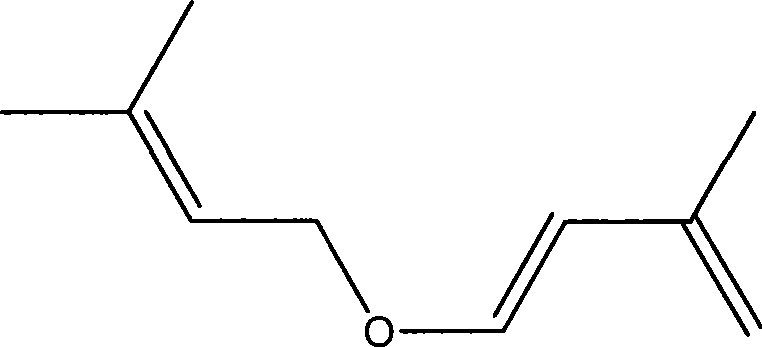
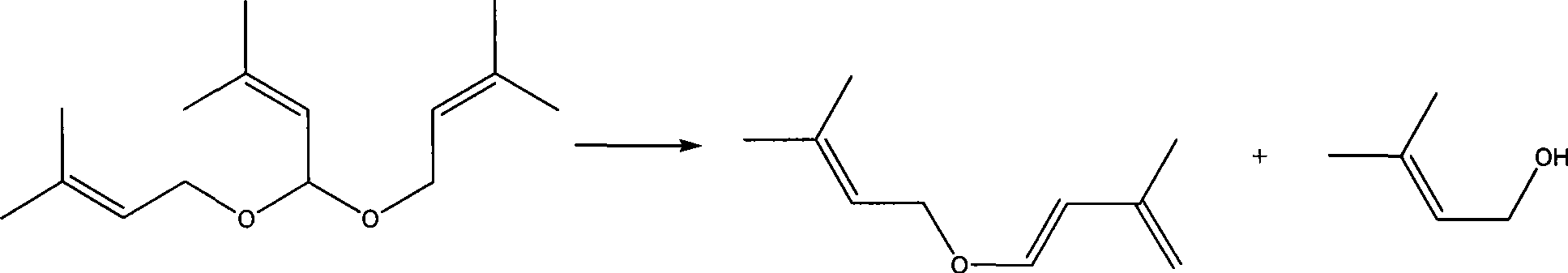
[0036] Example 3-5 Preparation of isoprenyl-3-methylbut-2-enyl ether
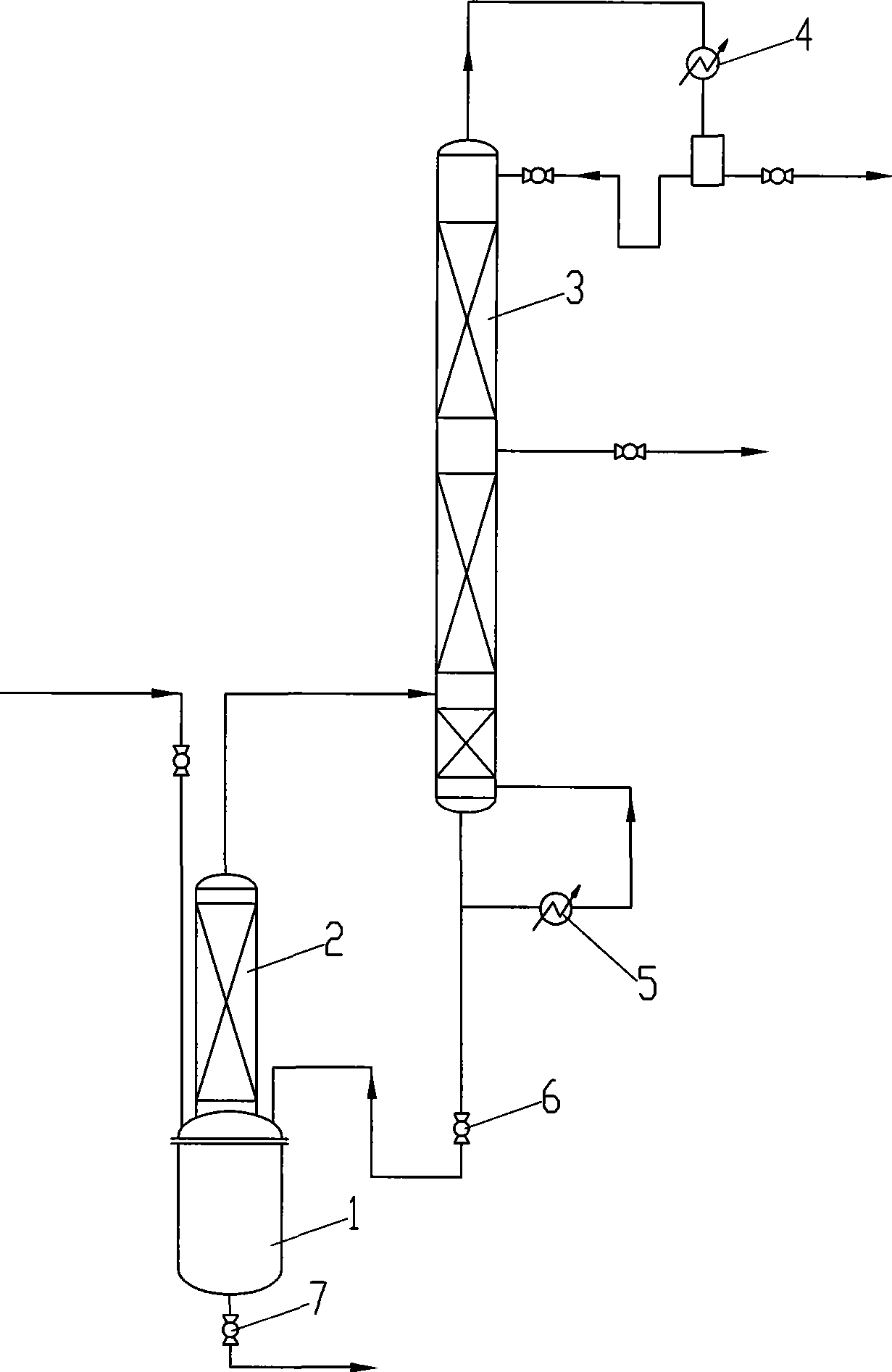
[0037]Use a reaction device with a gas-phase fixed-bed reactor, a 1.5m rectifying tower, and a 500ml reactor. The rectifying tower has a theoretical plate number of 30, and there is a side outlet at 15 plates from the bottom of the tower. The gas phase pipe at the top of the bed reactor leads to the bottom of the rectification tower, and the gas inlet is 1 plate away from the bottom of the rectification tower; Alkenyl acetal realizes accurate feeding; In the fixed-bed reactor, the catalyst prepared in embodiment 2 is loaded earlier, and 200g (content 97%) of 3-methylbut-2-enal diisoprene is dropped into the reactor Alkenyl acetal; pull vacuum at the top of the rectification tower, control 200-300MPa (absolute pressure); preheat the fixed bed to 200-250°C; heat up the reaction kettle, after heating to 140-150°C -2-enal diisoprenyl acetal is vaporized and enters the fixed bed reactor for reaction, the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com