Method for preparing iron oxalate
A technology of ferrous oxalate and ferrous sulfate, applied in the preparation of carboxylate, organic chemistry, etc., can solve the problems of low purity, difficult to ensure uniformity, low conductivity, etc., achieve simple and controllable process route, improve electrical The effect of chemical properties and high electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
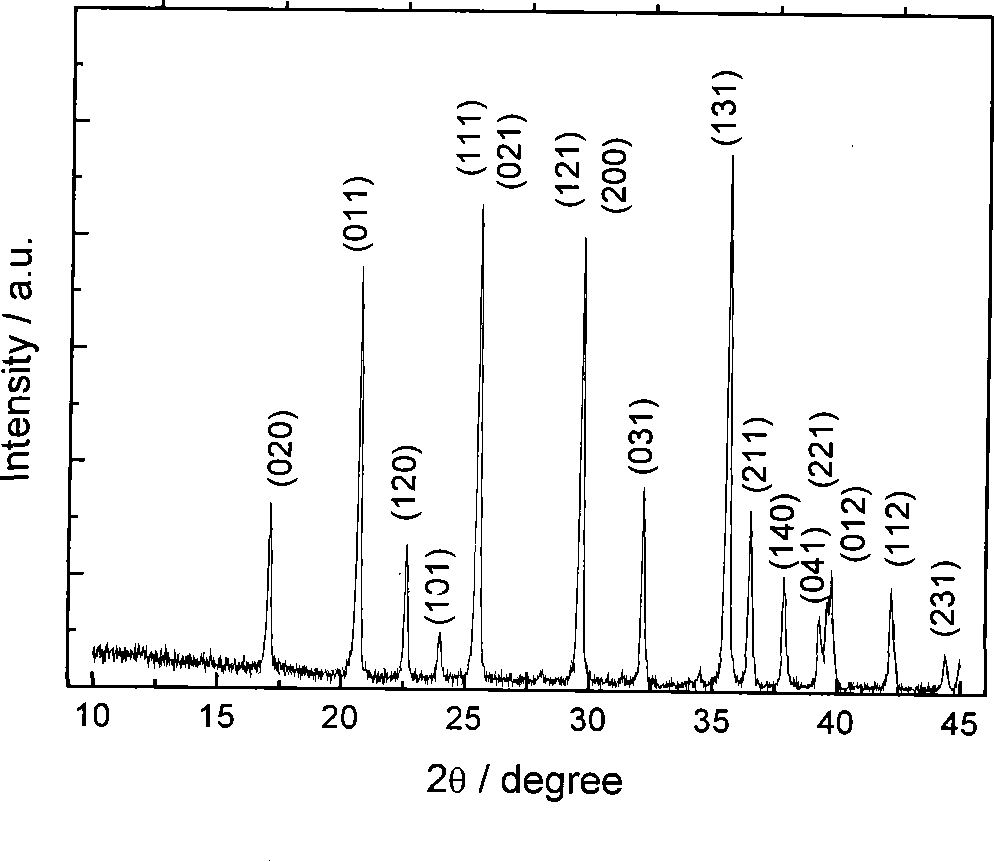
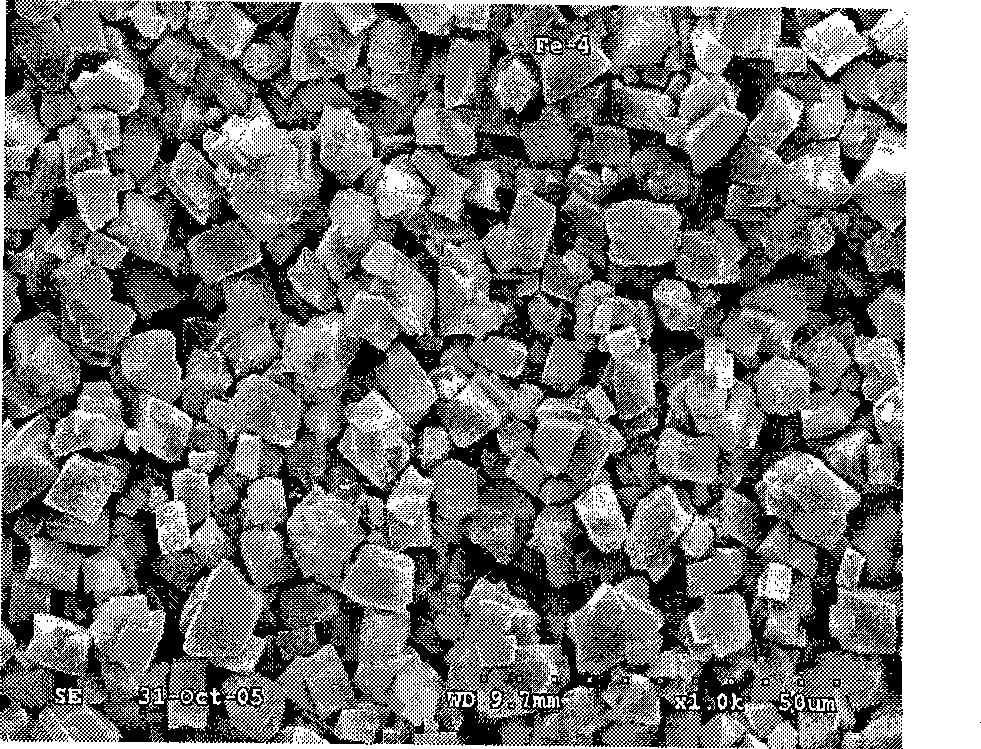
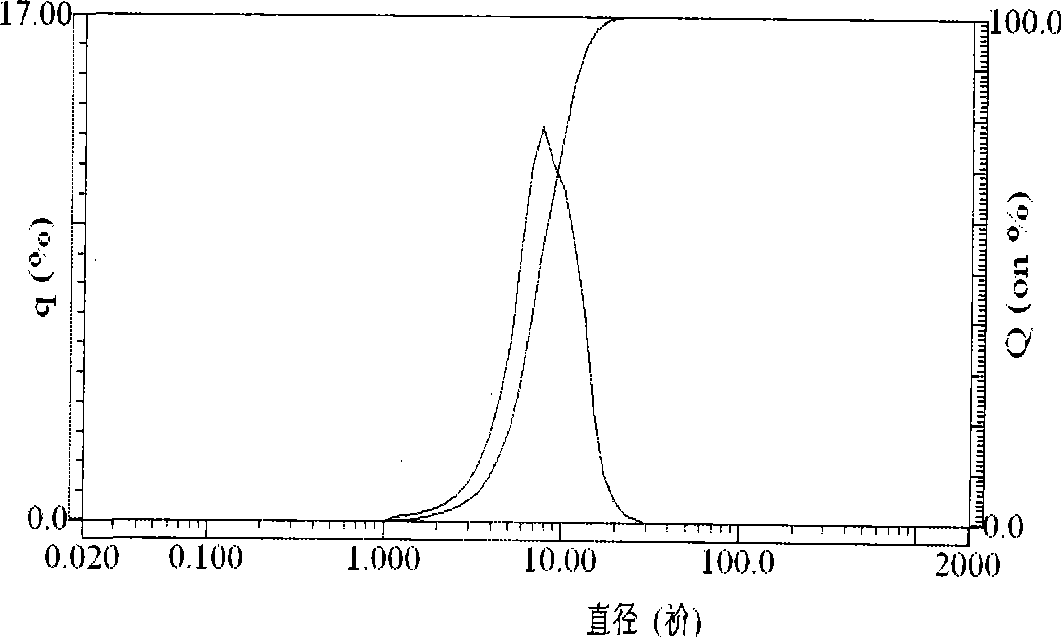
[0017] Example 1. Get 27.9g of industrial grade ferrous sulfate, dissolve it in 200ml of 50% dilute sulfuric acid, slowly add 10g of iron filings into the solution, stir, control the reaction temperature at 45°C, and obtain ferrous sulfate solution after suction filtration. Dissolve 11.1g of oxalic acid in 20ml of distilled water, stir and heat to 45°C, after the oxalic acid is completely dissolved, suction filter to obtain the oxalic acid solution. Then add the ferrous sulfate solution into the oxalic acid solution at a rate of 0.1 L / min, the reaction temperature is 65 degrees, keep stirring for 45 minutes, and then stand still for 10 to 30 minutes. The mother liquor was separated, washed twice with deionized water and then dried to obtain light yellow ferrous oxalate powder. Its particle size distribution and electrochemical performance are shown in figure 1 , figure 2 , image 3 .
Embodiment 2
[0018] Example 2. Take 27.9g of industrial-grade ferrous sulfate, dissolve it in 200ml of dilute sulfuric acid with a concentration of 50%, slowly add 10g of iron filings to the solution, stir, control the reaction temperature at 50°C, and add to the ferrous sulfate solution after suction filtration 1g of nickel sulfate (chemically pure), stirred and dissolved to obtain ferrous sulfate solution containing nickel sulfate composition. Dissolve 5.55g of oxalic acid and 6.26g of ammonium oxalate in 20ml of distilled water, stir and heat to 60°C, after the oxalic acid and ammonium oxalate are completely dissolved, suction filter to obtain a mixed solution of oxalic acid and ammonium oxalate. Then add the ferrous sulfate solution containing nickel sulfate to the oxalic acid and ammonium oxalate solution at a rate of 0.2 L / min, the reaction temperature is 60 degrees, keep stirring for 60 minutes, and then stand still for 10 to 30 minutes. The mother liquor is separated, washed twice...
Embodiment 3
[0019] Example 3. Take 41.9g of industrial-grade ferrous sulfate, dissolve it in 300ml of dilute sulfuric acid with a concentration of 50%, slowly add 15g of iron filings to the solution, stir, control the reaction temperature at 50°C, and add to the ferrous sulfate solution after suction filtration The cupric sulfate of 0.5g and 0.5g magnesium sulfate (chemically pure), make it dissolve with stirring and make the ferrous sulfate solution that contains copper sulfate and magnesium sulfate. Dissolve 11.1g of oxalic acid in 20ml of distilled water, stir and heat to 65°C, after the oxalic acid is completely dissolved, suction filter to obtain the oxalic acid solution. Then add the ferrous sulfate solution containing copper sulfate and magnesium sulfate into the oxalic acid solution at a rate of 0.2 L / min, the reaction temperature is 50°C, keep stirring for 60 minutes, and then stand still for 10 to 30 minutes. The mother liquor is separated, washed twice with deionized water and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com