Alkyl imidazoles perrhenate ion liquid and preparation method thereof
A perrhenate, ionic liquid technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., can solve the instability of air and moisture, limit applications and other problems, to achieve the effect of protecting the environment, shortening the reaction time, and simplifying the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
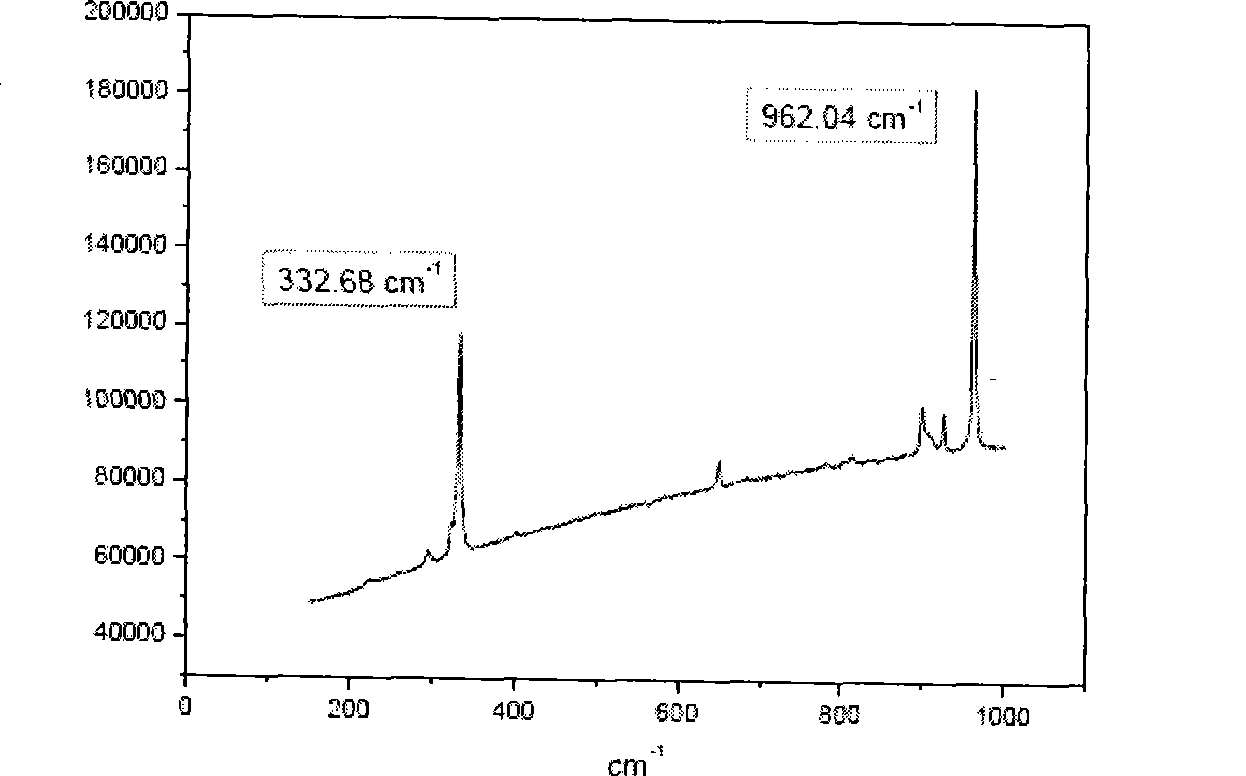
Image
Examples
Embodiment 1
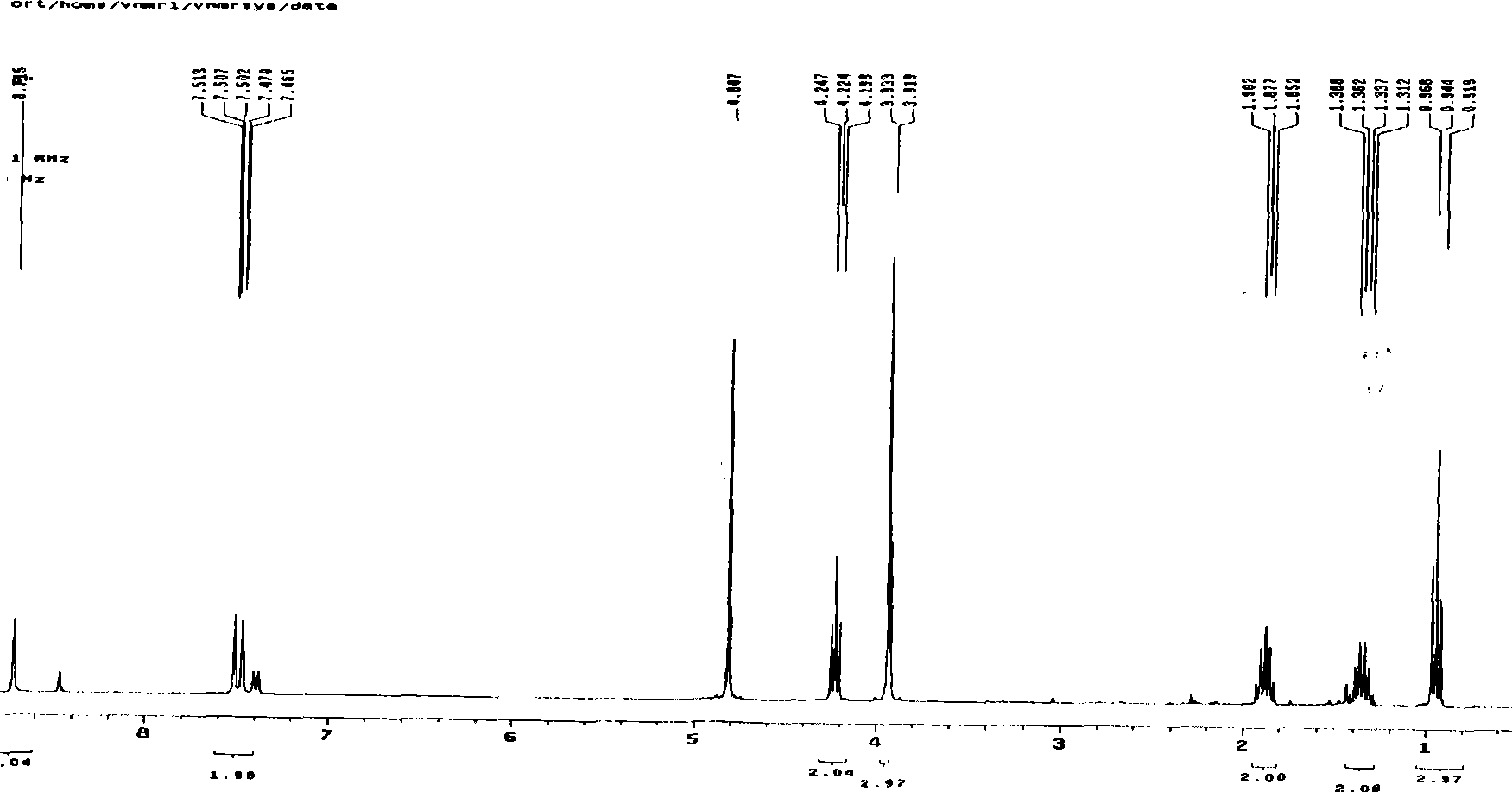
[0016] Embodiment 1 1-butyl-3-methyl-imidazole perrhenate ([Bmim][ReO 4 ]) ionic liquid
[0017] One) the structural formula is as follows:
[0018]
[0019] Two) preparation method is as follows:
[0020] (1) Synthesis of chloro-1-butyl-3-methyl-imidazole (BMIC):
[0021] Collect n-chlorobutane and N-methylimidazole in a distillation apparatus at 78°C and 188.5°C respectively to obtain pure products.
[0022] Take n-chlorobutane and N-methylimidazole with a molar ratio of 1.2:1, heat and reflux at 80°C for 72 hours, place it at 0-10°C for 7-9 hours, and obtain a white product after filtration , which is 1-butyl-3-methylimidazole chloride (BMIC).
[0023] (2) Purification of chlorination-1-butyl-3-methyl-imidazole (BMIC)
[0024] Add ethyl acetate and acetonitrile solution with a volume ratio of 2:1 to the above white product, and heat to reflux in an oil bath at 75°C to completely dissolve the white product. Cool at 0~-10°C, place for 7~9 hours, and obtain white crys...
Embodiment 2
[0033] Embodiment 2 1-ethyl-3-methyl-imidazole perrhenate ([Emim][ReO 4 ]) ionic liquid
[0034] One) the structural formula is as follows:
[0035]
[0036] Two) preparation method is as follows:
[0037] (1) Conversion of Hydroxide-1-Ethyl-3-Methyl-Imidazole (EMIOH)
[0038] Take a certain amount of chlorinated-1-ethyl-3-methylimidazole, add 2 to 4 times of deionized water to dissolve, add it to OH type anion exchange resin for ion exchange, collect the outflowing solution until chlorine is detected ions, the collected solution is the EMIOH solution;
[0039] (2) Take the hydroxide-1-ethyl-3-methyl-imidazole solution, carry out accurate titration with hydrochloric acid of known concentration, and calculate the concentration of the collected EMIOH solution. Add hydroxide-1-ethyl-3-methyl-imidazole to ammonium perrhenate aqueous solution, and the molar ratio of hydroxide-1-ethyl-3-methyl-imidazole to ammonium perrhenate is 1:1 After heating and stirring at 70°C for 3 h...
Embodiment 3
[0041] Example 3 1-propyl-3-methyl-imidazole perrhenate ([Tmim][ReO 4 ]) ionic liquid
[0042] One) the structural formula is as follows:
[0043]
[0044] Two) preparation method is as follows:
[0045] (1) Conversion of Hydroxide-1-Propyl-3-Methyl-Imidazole (TMIOH)
[0046] Take a certain amount of bromide-1-propyl-3 methylimidazole, add 2 to 4 times of deionized water to dissolve, add it to OH type anion exchange resin for ion exchange, collect the outflowing solution until bromide ion is detected So far, the collected solution is the TMIOH solution;
[0047] (2) Take the hydroxide-1-propyl-3-methyl-imidazole solution, carry out accurate titration with hydrochloric acid of known concentration, and calculate the concentration of the collected TMIOH solution. Add hydroxide-1-propyl-3-methyl-imidazole to ammonium perrhenate aqueous solution, the molar ratio of hydroxide-1-propyl-3-methyl-imidazole to ammonium perrhenate is 1:2, After heating and stirring at 70°C for 5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com