Recombinant bovine enterokinase, preparation method and use thereof
A bovine enterokinase and heavy chain technology, applied in the biological field, can solve problems such as low protein activity and difficult renaturation of inclusion body products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Cloning of bovine EK cDNA fragment
[0039] Take 150 mg fresh bovine duodenum, extract total RNA with Trizol reagent (product of Invitrogen), take 0.5 μl total RNA, add 3 μl oligo(dT)18 primer, add water to a total volume of 10.5, and mix well. Place at room temperature for 10 minutes, and centrifuge at high speed for 5 minutes. Then add 4.0 μl 5×reverse transcription reaction buffer, 0.5 μl RNase inhibitor, 2.0 μl 10mMdNTP, 2.0 μl DTT, 1 μl MMLV reverse transcriptase, mix well, and react at 37°C for 120min. Take 1.5 μl RT product, add 100 pmol upstream primer (5'-aagcttatggggtcaaagcgaagtgt-3') and downstream primer (5'-gaattctcaatgtagaaaactttgtatcc-3', design primer with reference to the coding sequence of bovine Enterokinase in CenBank, sequence number: U09859), 2.5 μl 10 ×PCR buffer, 2μl 2.5mMd NTP, 2.5DMSO, 0.25Taq enzyme, add water to a final volume of 25μl, mix well and quickly add 5μl paraffin oil for PCR amplification. The reaction conditions were dena...
Embodiment 2
[0040] Example 2 Design of EKLm and construction of pET39b-EKL and pET39b-EKLm expression plasmids
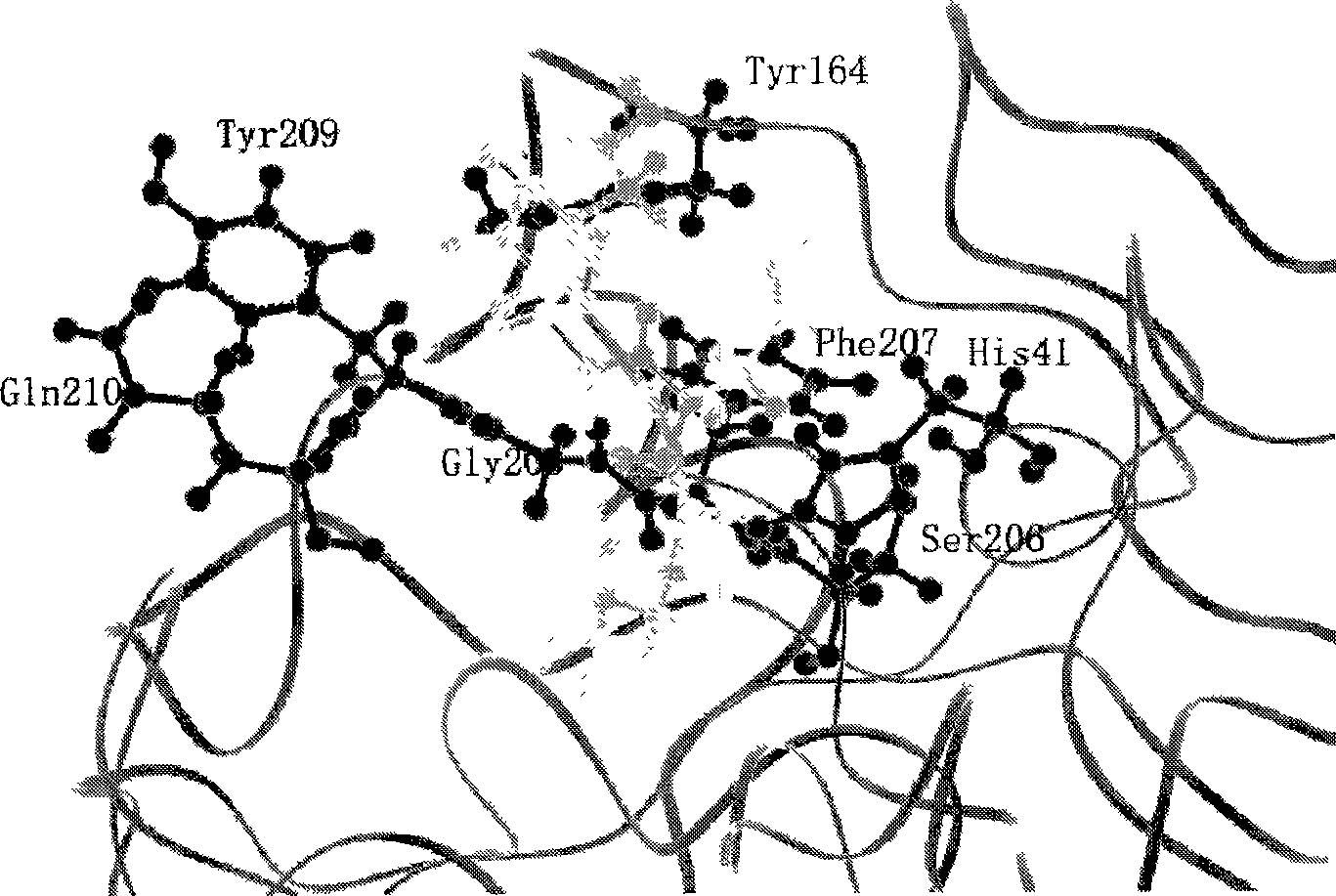
[0041] In order to improve the activity of enterokinase, the inventors hereby refer to bovine enterokinase light chain and its inhibitor VD 4 The crystal structure of the complex of K-chloroform (Code no: 1EKB, Protein Dtabase Bank), observed by VD 4 K is the center 5 In the case of amino acid residues within the range, select the residues that may enhance the binding activity of EKL and DDDDK after mutation, and mutate it into R to enhance its binding activity with VD 4 The binding force of D in K. A total of 9 mutants were designed, such as figure 1 shown.
[0042] Using the correctly identified pMD18-T-EK as a template, the cDNA fragment of the light chain coding region of bovine enterokinase was amplified by PCR. The upstream primer has an EcoRI restriction site and an enterokinase recognition site, and the downstream primer has a HindIII restriction site and a stop c...
Embodiment 3
[0043] Example 3 Induced expression of pET39b-EKL and pET39b-EKLm
[0044] The thioredoxin analog DsbA provided on the pET39b vector can help the protein to fold normally, thereby producing an active soluble protein. Each engineered bacteria was inserted into LB medium. Place in a shaker at 37°C and 250rpm for overnight culture. The overnight seed solution was added to the 2YT medium, and placed in a shaker for cultivation at 37° C. and 250 rpm. When OD 600 When the temperature is 0.6, 0.1 mM IPTG is added to induce, and placed in a shaker to continue culturing at 30°C and 250 rpm. After 2 hours of induction, samples were taken, centrifuged to remove the supernatant, and the precipitate was placed in the refrigerator. Then samples were taken every 1 hour, and the supernatant was also centrifuged to remove, and the precipitate was placed in the refrigerator. After 6 hours of induction, the bottle was closed, and the supernatant was removed by centrifugation. The induced ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com