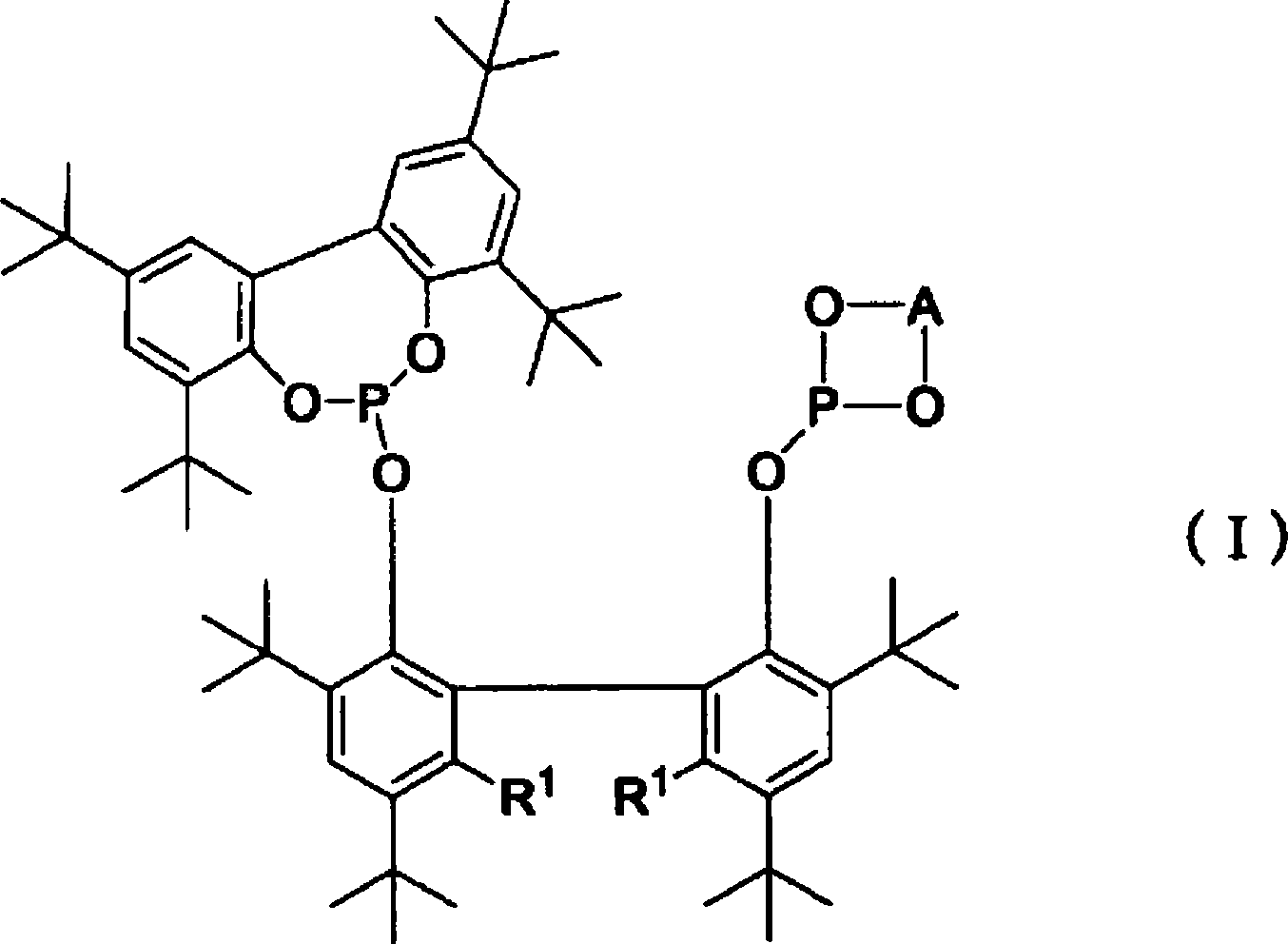
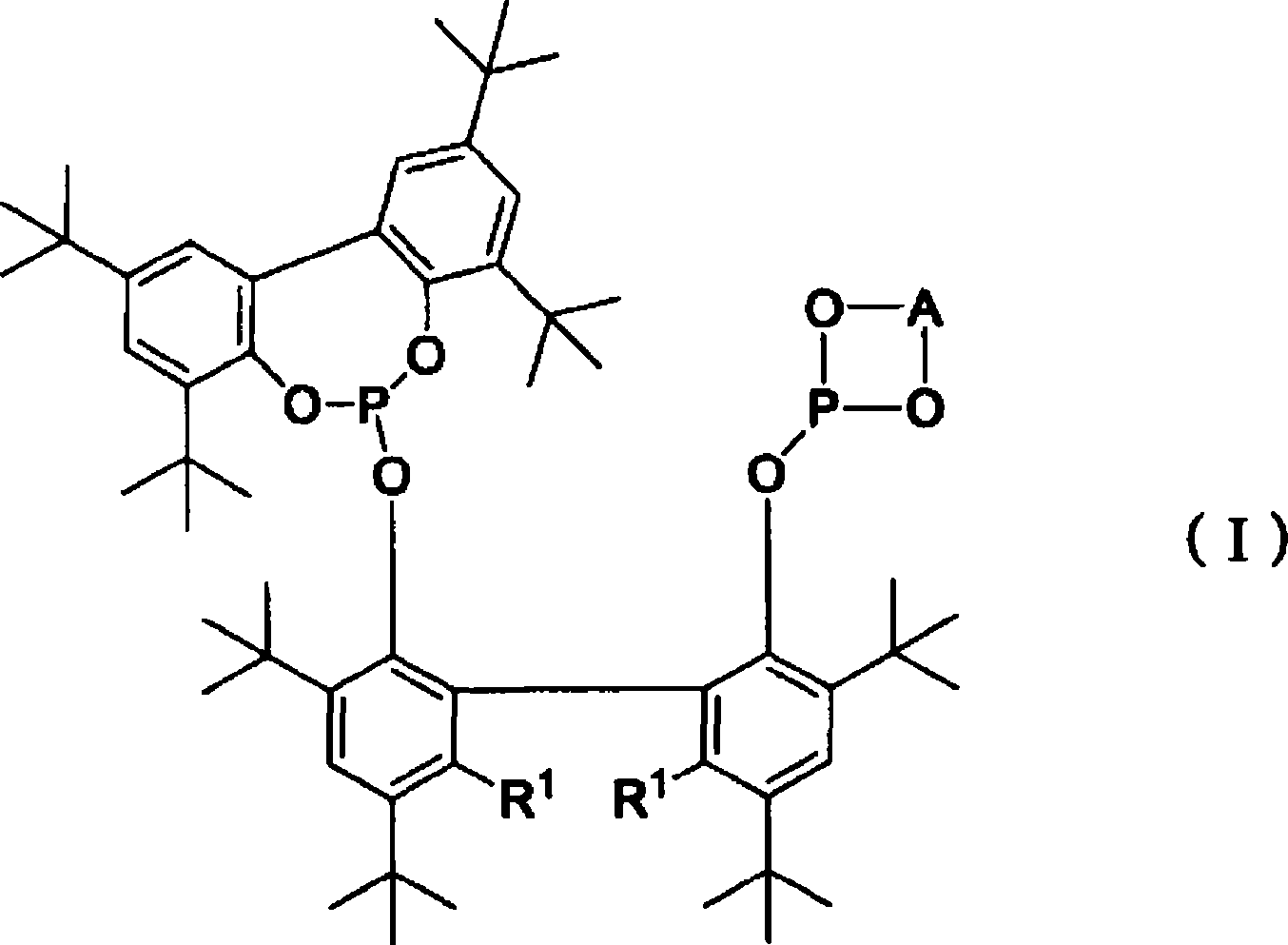
Method for producing aldehyde using bisphosphite and group 8-10 metal compound, and such bisphosphite
A bisphosphite and carbon double bond technology, applied in the field of aldehyde preparation, can solve the problems of reduced target aldehyde yield and inability to inhibit isomerization reaction, etc., to maintain catalyst activity, maintain productivity, hydrolysis resistance or The effect of high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The production method of bisphosphite (I) is not particularly limited, for example, there are the following methods.
[0039] R 1 When representing a hydrogen atom, the bisphenol represented by the formula (1-1) [hereinafter referred to as bisphenol (1- 1)] with general formula PY 1 3 (Y 1 Represent chlorine atom, bromine atom or iodine atom) shown in phosphorus trihalide compound reaction, thus prepare monophosphite shown in formula (3-1) [hereinafter referred to as monophosphite (3-1)] ( This method is hereinafter referred to as "monophosphite production method (a)").
[0040] [chemical formula 7]
[0041]
[0042] [chemical formula 8]
[0043]
[0044] On the other hand, R 1 When expressing an alkyl group, first, under an inert gas atmosphere such as nitrogen and argon, in the presence of a solvent and an alkaline substance used as needed, the bisphenol compound represented by the general formula (1-2) [hereinafter referred to as bisphenol (1-2)] react ...
Embodiment 1
[0128] [chemical formula 15]
[0129]
[0130] Add 82.12g (200mmol) 4,4',6,6'-tetra-tert-butyl-2,2'-bisphenol and 500mL toluene into a 1000mL three-necked flask with a thermometer and a dropping funnel, then add 59.2 g (390mmol) triethylamine, system nitrogen replacement. Next, 11.4 mL (130 mmol) of phosphorus trichloride was added dropwise over 30 minutes while maintaining the internal temperature at 20-30° C., and after the dropwise addition was completed, it was further stirred at room temperature for 12 hours. After the reaction is terminated, the by-product triethylamine hydrochloride is removed by filtration, and toluene and triethylamine (50° C. / 0.01 MPa) are decompressed from the resulting filtrate to obtain 95.0 g of crude monophosphite (3 -1). It was recrystallized and purified from a mixed solvent of 300 mL of acetonitrile and 150 mL of tetrahydrofuran to obtain 82.80 g of monophosphite (3-1) (based on phosphorus trichloride, the yield was 75%, and the purity w...
Embodiment 2
[0132] [chemical formula 16]
[0133]
[0134] Add 8.49g (10mmol) of monophosphite (3-1) and 50mL of toluene to a 100mL three-necked flask with a thermometer and a dropping funnel, and then add 1.52g (15mmol) of triethylamine to replace the system with nitrogen. Next, 2.6 mL (30 mmol) of phosphorus trichloride was added dropwise over 30 minutes while the internal temperature was kept at 20-30° C. After the dropwise addition, the temperature was raised to 70° C. and further stirred for 12 hours. After returning to room temperature, by-product triethylamine hydrochloride was removed by filtration, and phosphorus trichloride, toluene and triethylamine (50°C / 0.01MPa) were distilled off under reduced pressure from the obtained filtrate, thereby obtaining 10.5 g of crude Halophosphite (5-1).
[0135] [chemical formula 17]
[0136]
[0137] Then, add 10.5 g of the crude halogenated phosphorous acid ester (5-1), 100 mL of toluene and 3.03 g (30 mmol) of triethylamine obtained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com