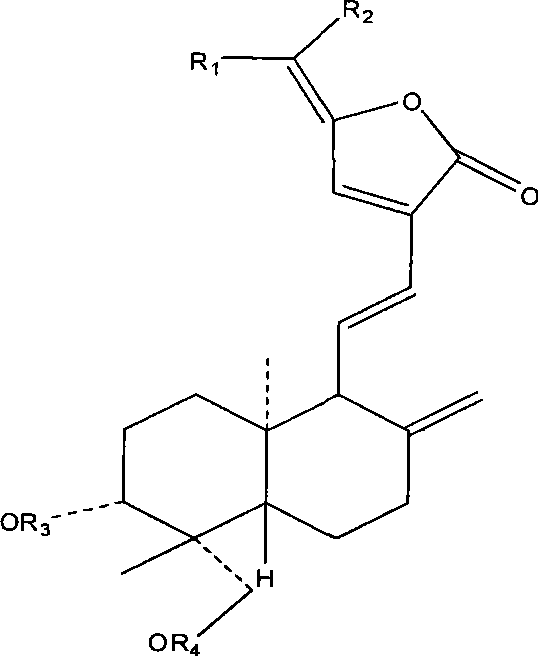
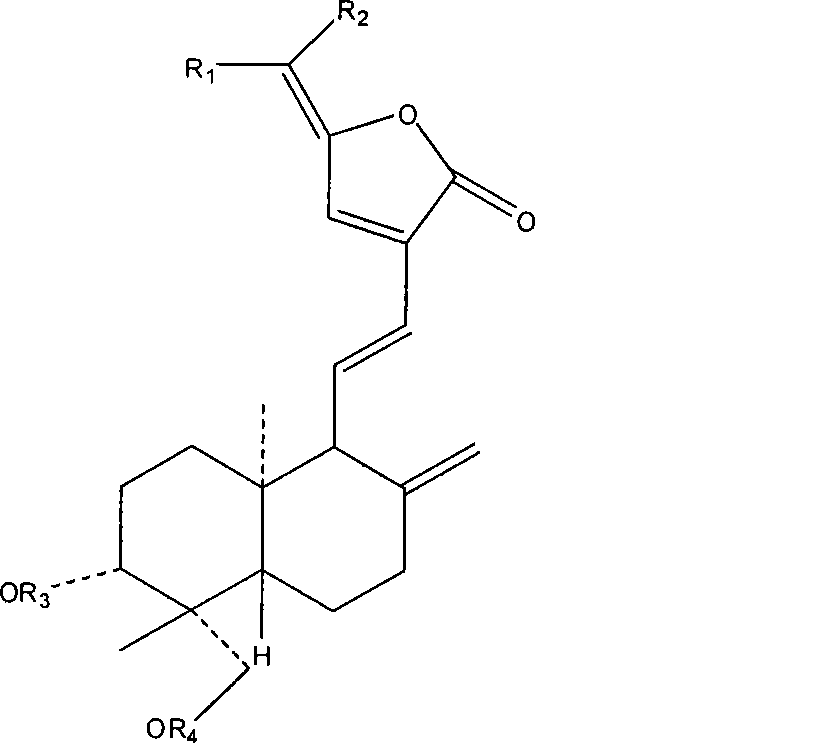
Use of 15-methano-substituted-andrographolide derivative in preparing anti-inflammatory ntipyretic analgesic medicine
A technology of andrographolide and methylene, which is applied in the fields of anti-inflammatory, antipyretic and analgesic effects, can solve problems such as no related reports, achieve the effect of improving oral efficacy, expanding the range of options, and avoiding side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1 Pharmacological activity of 15-methylene substituted andrographolide derivatives
[0013] 1. Anti-inflammatory experiment
[0014] 1. Experiment on the effect of p-xylene on mouse ear swelling
[0015] The experimental drugs were drug samples of the present invention, andrographolide provided by Beijing Institute of Medical Engineering Biotechnology, and indomethacin (indomethacin enteric-coated tablets; batch number: 20060502) produced by Shanxi Yunpeng Pharmaceutical Co., Ltd. as a positive control. The drug was prepared by using 0.1% Tween-80 (Tianjin Kemiou Chemical Reagent Development Center, batch number: 20030720) to aid dissolution, and 0.5% sodium carboxymethylcellulose (Shanghai Chemical Reagent Company, batch number: F20020928) for suspension. The experimental animals were clean-grade male Kunming mice (certificate number: 0009255) with a body weight of 20±2 g provided by the Experimental Animal Center of Henan Province. The inflammatory agent xyl...
Embodiment 2
[0046] The acute toxicity of embodiment 2 ADD-3
[0047] Animals: clean-grade Kunming mice, weighing 20±2 g, half male and half male, certificate number: 0009898, provided by the Experimental Animal Center of Henan Province.
[0048] Drugs: 15-methylene substituted andrographolide derivatives of the present invention.
[0049] Experimental method: Take 20 mice weighing 20±2g, half male and half male, and randomly divide them into ADD-3 low-dose group and ADD-3 high-dose group, with 10 mice in each group. After the animals were fasted for 12 hours (drinking water was not restricted), the doses of 2.00 g / kg and 5.00 g / kg of ADD-3 were given by intragastric administration respectively, and the intragastric volume was 0.2 mL / 10 g. Observe and record the poisoning performance of the animals. Weigh once a week. Continuous observation for 14 days. The results are shown in Table 6.
[0050] Experimental results: The mice did not show obvious signs of poisoning and did not die, in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com