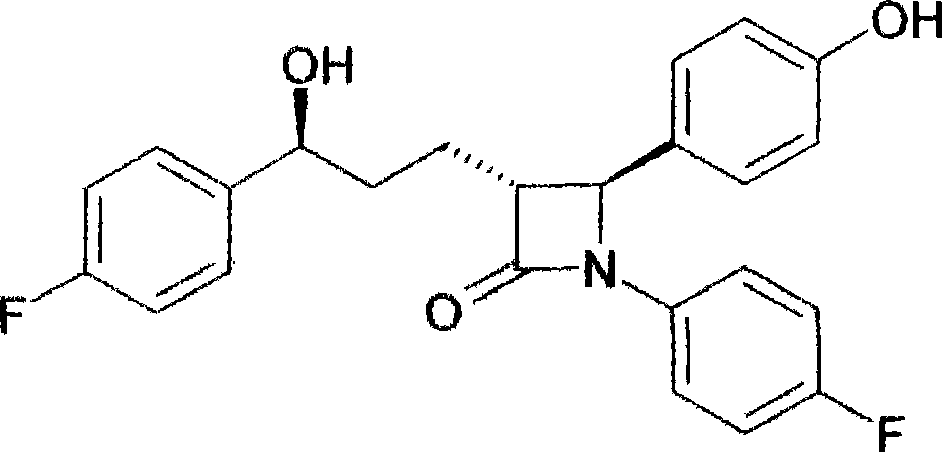
Novel preparation method of ezetimibe
A technology of ezetimibe and compounds, applied in the preparation of cholesterol absorption inhibitors, in the field of ezetimibe, which can solve problems such as high cost, unsuitable for industrial production, and weak hydroxyl activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
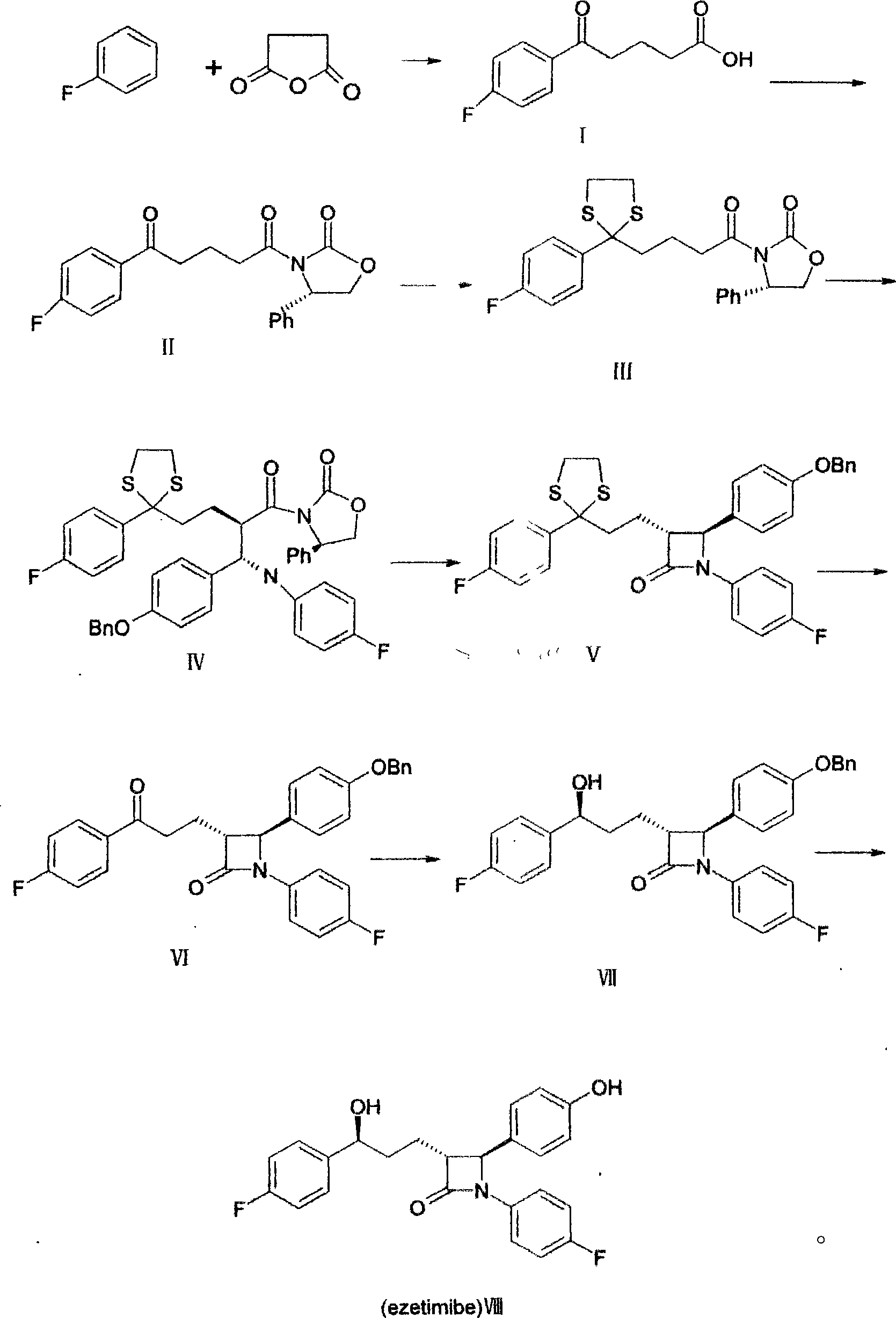
[0032] Mix 71.4 g of aluminum trichloride and 200 ml of fluorobenzene, add a solution of glutaric anhydride (28.6 g) in fluorobenzene (100 ml) dropwise at 20°C, and react at 30°C for 3 hours after dropping. Cool to about 0°C, add dropwise 200ml of 1M hydrochloric acid, the temperature does not exceed 20°C. Add a large amount of ice water after dropping, stir, filter, and wash the filter cake. Add the filter cake to 800 ml of saturated sodium bicarbonate solution, stir at room temperature for 1 h, filter, and add activated carbon to the filtrate for decolorization. Add concentrated hydrochloric acid to adjust the pH value to 1, filter and wash the filter cake, and dry to obtain a white solid. Namely compound I. Yield 71%.
Embodiment 2
[0034] Mix 47g of compound I, 60ml of triethylamine and 250ml of dichloromethane, dropwise add 27.6ml of pivaloyl chloride, react for 3h, add (4s)-4-phenyl-2-oxazolidinone 38g, DMF25ml, 4, 4-Dimethylaminopyridine 4g, reflux reaction for 10h, after cooling, add 200ml of 5M hydrochloric acid dropwise, oscillate and mix well, then let stand, and separate the organic layer. It was washed successively with saturated sodium bicarbonate solution and water, and dried over anhydrous sodium sulfate. The filtrate was concentrated to dryness to obtain a white solid, namely compound II, 49 g in total, with a yield of 61%.
Embodiment 3
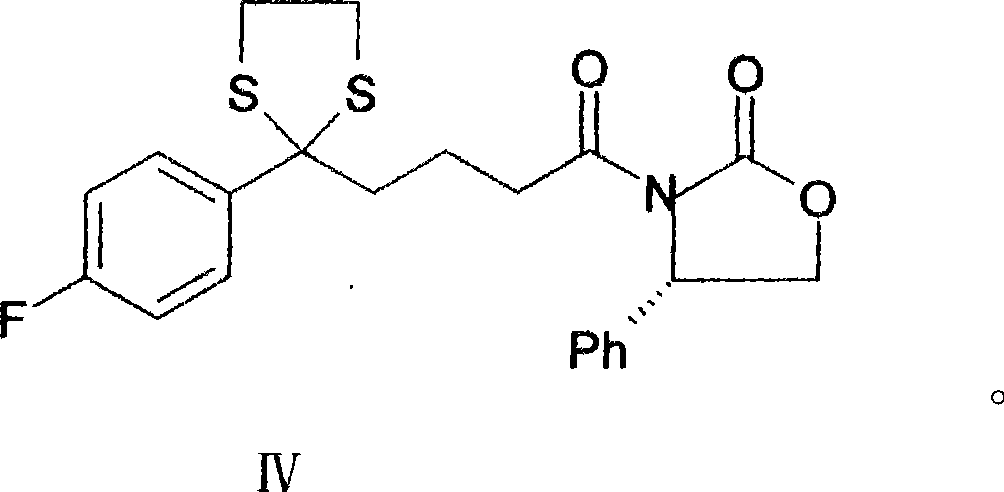
[0036] Put 15g of compound II and 200ml of toluene in a mixing tank equipped with a water separator, add 2ml of titanium tetrachloride and 4.2ml of thiodiethanol in sequence, stir, and heat up to reflux. After reacting for 6 hours, it was cooled to room temperature and washed with an equal volume of saturated brine. Then cooled to -10°C for crystallization. Dry to obtain a white solid, which is Compound III, 14.9 g in total, with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com