Process for producing potassium permanganate doped potassium ferrate
A technology of potassium permanganate and potassium ferrate, applied in chemical instruments and methods, iron compounds, inorganic chemistry, etc., can solve the problems of poor stability of potassium ferrate, low yield of potassium ferrate, thermal decomposition, etc. The effect of good chemical properties, high discharge specific capacity, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
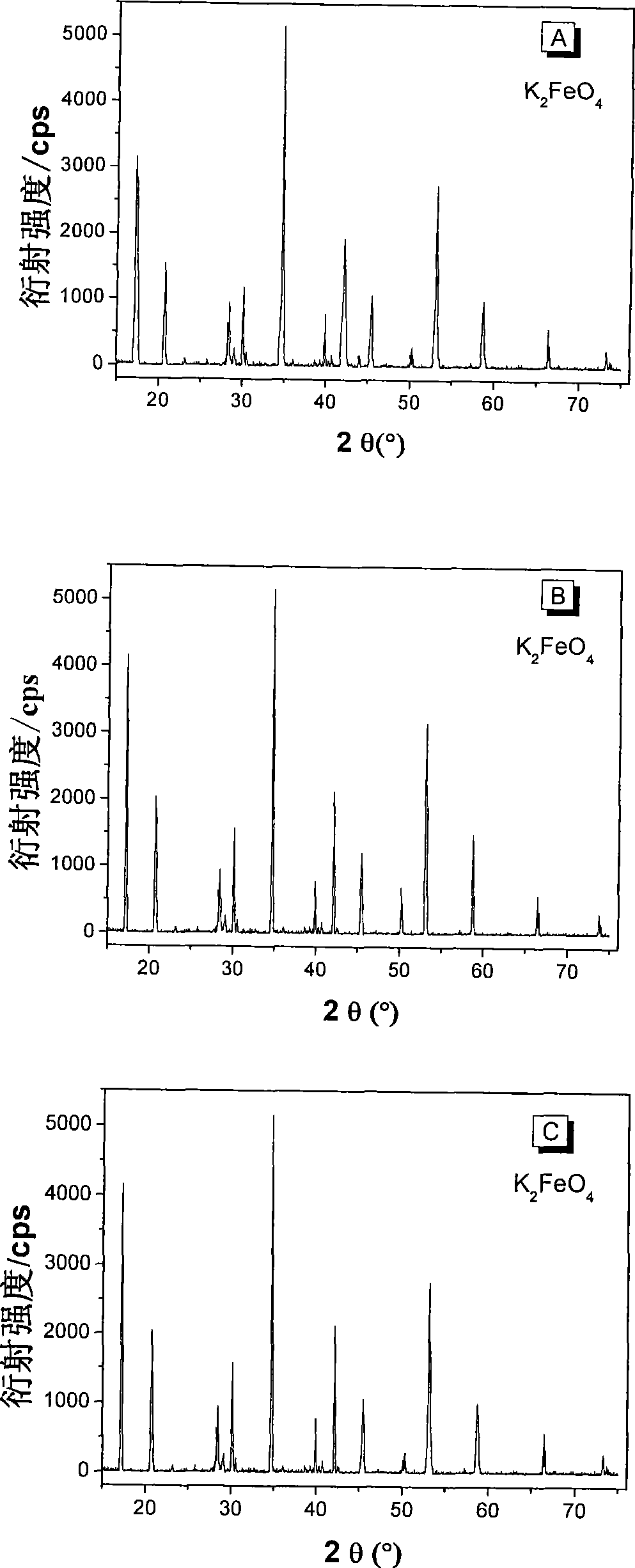
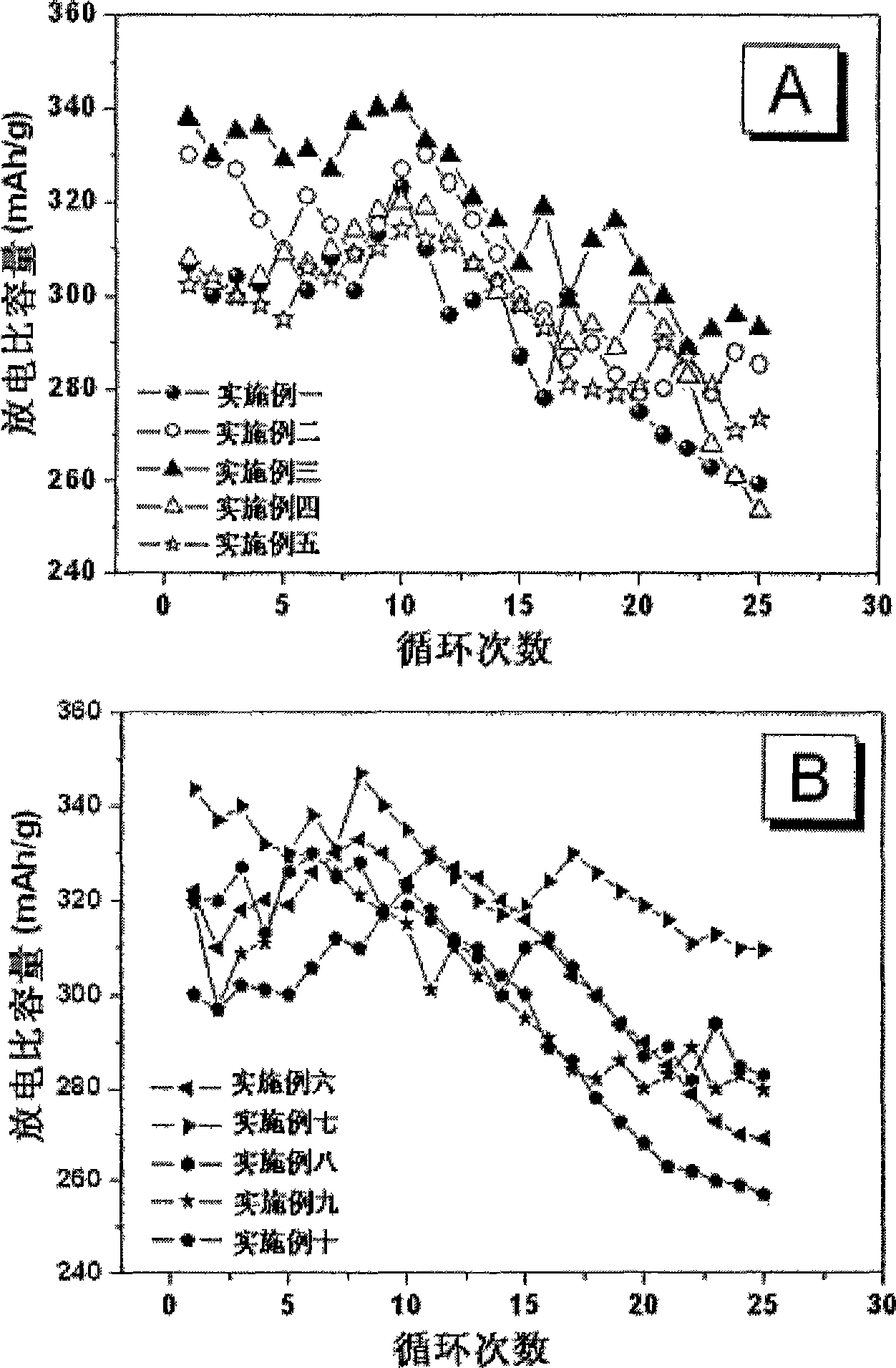
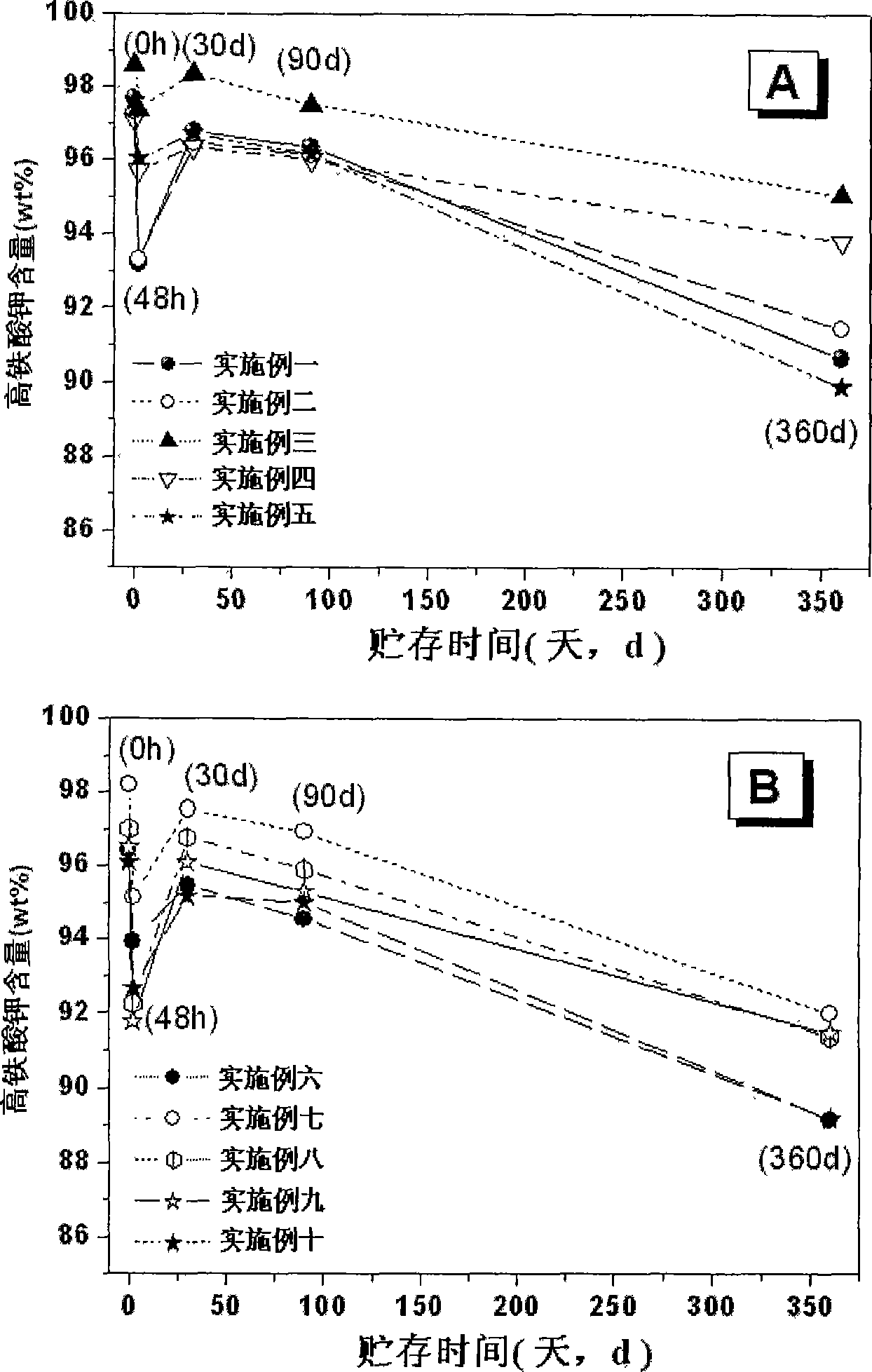
Embodiment 1
[0033] The first step mixes ferric nitrate and sodium silicate uniformly in a ratio of 1:0.005 in molar ratio of iron to sodium silicate;
[0034] The second step is that the above mixture is dissolved in a concentration of 3.0M potassium hydroxide solution;
[0035] In the third step, under the condition of stirring, the potassium hypochlorite solution containing the same concentration of KOH as the second step is added to the above potassium hydroxide solution in a ratio of 1:3 by the molar ratio of Fe and potassium hypochlorite, and the temperature is controlled at 0°C. React for 10 minutes, filter under reduced pressure under the condition of less than 100Kpa, and separate the purple suspended solid product prepared by the reaction;
[0036] The fourth step dissolves the above-mentioned purple solid product in a mixed solution of potassium hydroxide (concentration is 3.0M) and potassium permanganate (concentration is 5.0g / L) whose volume is 0.2 times the volume of the reac...
Embodiment 2
[0040] The first step ferric chloride and cupric chloride are mixed evenly in the ratio of 1:0.008 by the mol ratio of ferric ion and copper ion;
[0041] The second step is that the above mixture is dissolved in a concentration of 4.0M potassium hydroxide solution;
[0042] In the third step, under the condition of stirring, add the sodium persulfate solution containing the same concentration KOH as the second step in the ratio of 1:4 by the molar ratio of Fe and sodium persulfate to the above potassium hydroxide solution, and the temperature is controlled at 45 ℃, react for 20 minutes, separate the purple suspended solid product prepared by the reaction, and filter under reduced pressure under the condition of less than 100Kpa;
[0043] In the 4th step, the above-mentioned purple solid product is mixed with a mixed solution of potassium hydroxide (concentration is 12.0M) and potassium permanganate (concentration is 1.0g / L) that is 0.25 times the volume of the reaction system...
Embodiment 3
[0047] The first step mixes ferric nitrate and sodium silicate uniformly in a ratio of 1:0.01 in molar ratio between iron and sodium silicate;
[0048] The second step is that the above mixture is dissolved in a potassium hydroxide solution with a concentration of 6.0M;
[0049] In the third step, under the condition of stirring, the sodium hypochlorite solution containing the same concentration of KOH as the second step is added to the above potassium hydroxide solution in a ratio of 1:6 by the molar ratio of Fe and sodium hypochlorite, and the temperature is controlled at 25 ° C. The reaction is 45 Minutes, filter under reduced pressure less than 100Kpa, separate the purple suspended solid product prepared by the reaction;
[0050] In the 4th step, the above-mentioned purple solid product is mixed with a mixed solution of potassium hydroxide (concentration is 6.0M) and potassium permanganate (concentration is 25.0g / L) that is 1.5 times the volume of the reaction system solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition efficiency | aaaaa | aaaaa |
| decomposition efficiency | aaaaa | aaaaa |
| decomposition efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com