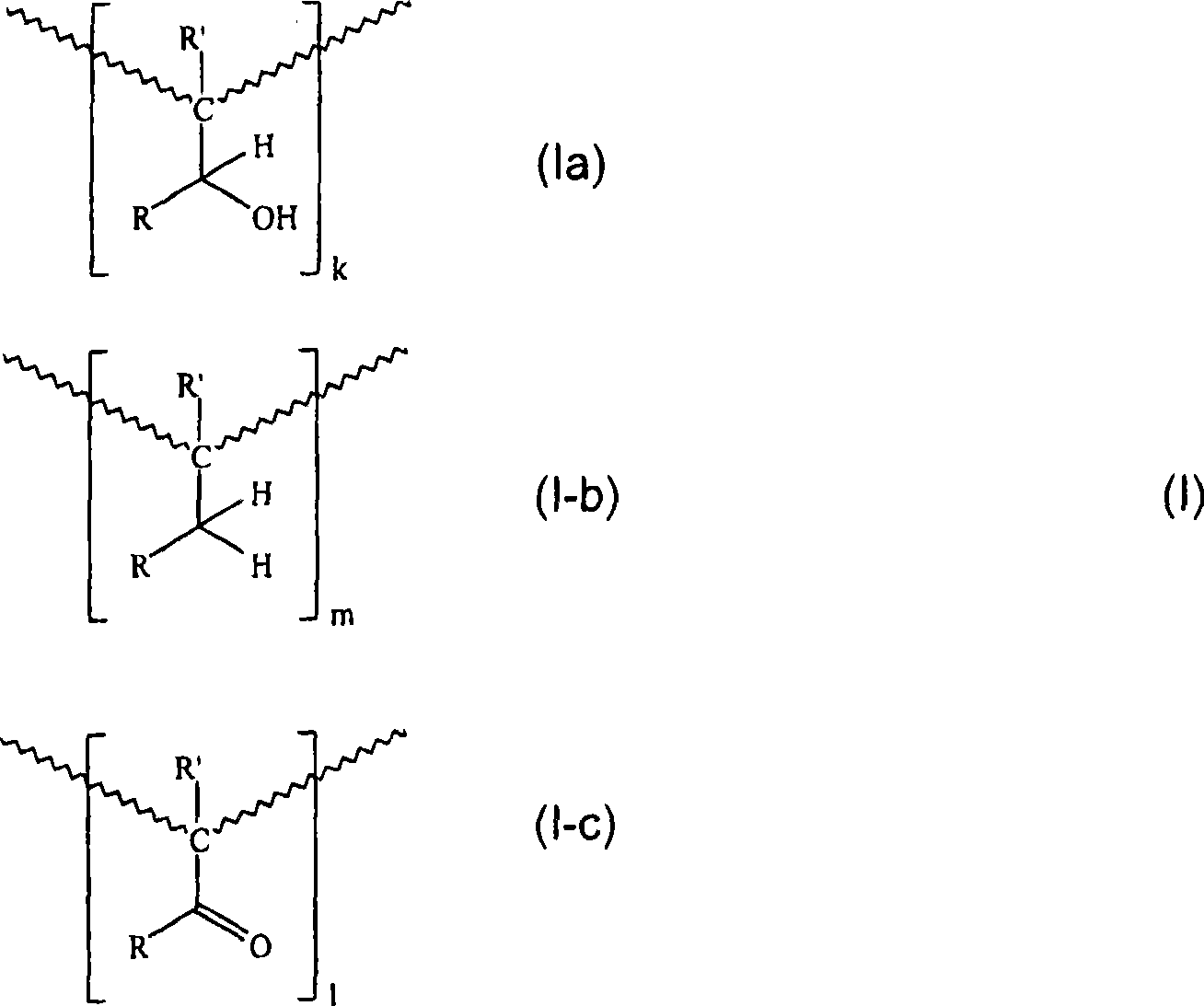
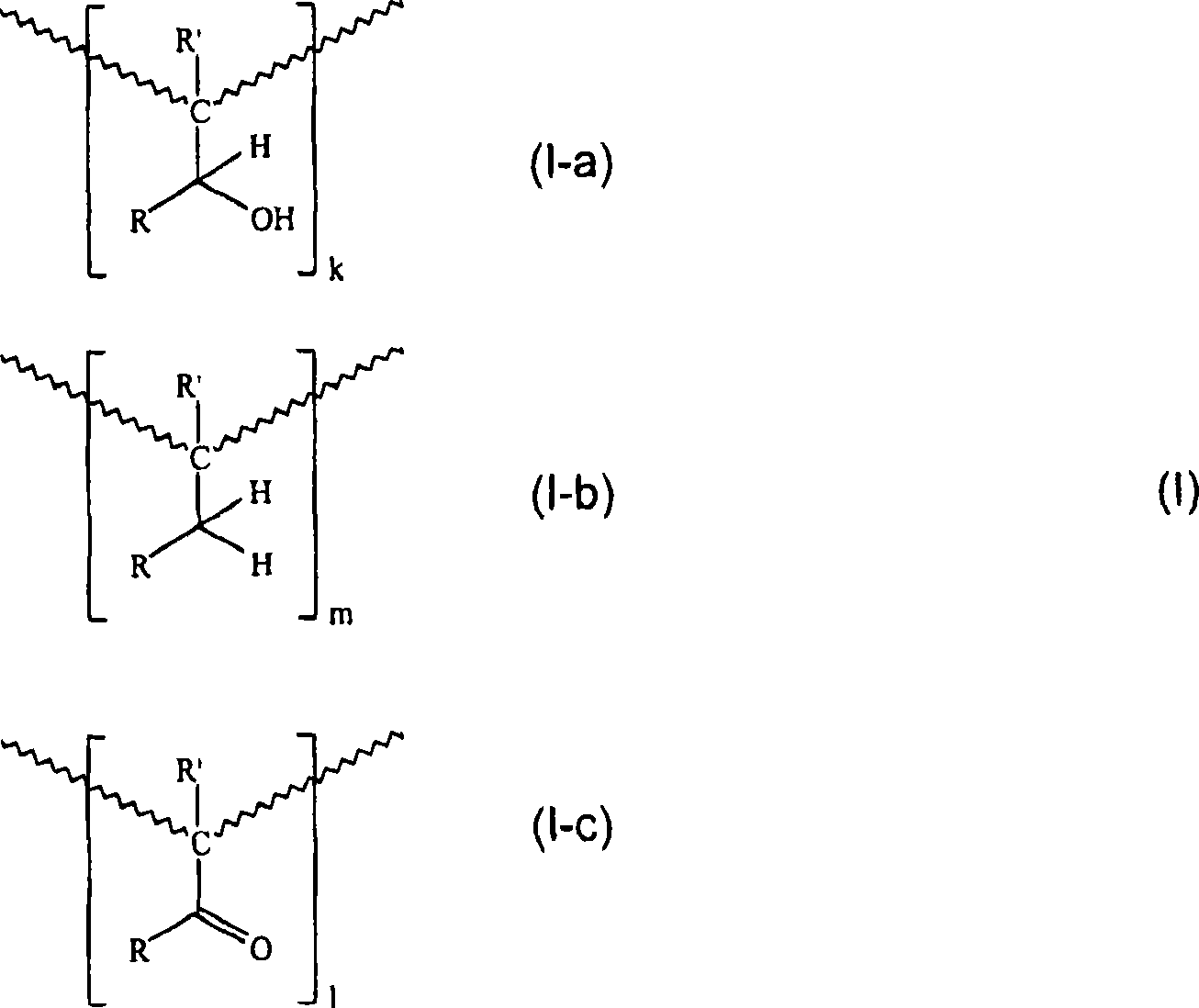
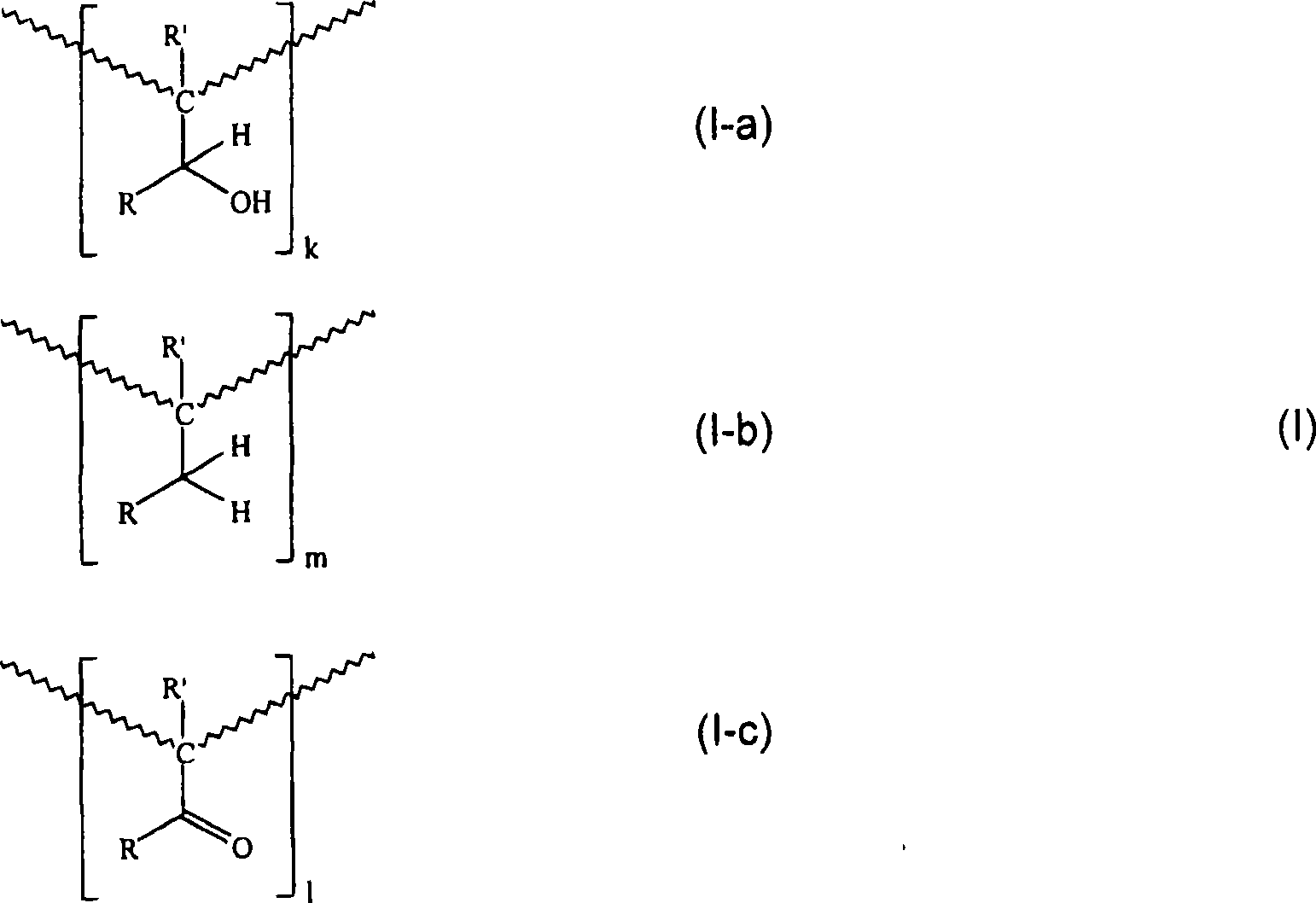Printing ink compositions
A technology of ink composition and compound, applied in the direction of ink, household utensils, applications, etc., can solve problems such as ink applications that are not described
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0140] Analytical method
[0141] Determination of free formaldehyde content
[0142] Determination of formaldehyde content by HPLC according to the Lutidin-Methode (official overview of research methods according to §64LFGB K 84.007 (EG): "Proof and Quantitative Determination of Free Formaldehyde")
[0143] Instrumentation: HPLC system equipped with two isocratic elution pumps (isokratischen Pumpen), thermostatted reactor, variable UV / VIS detector and analytical unit (eg Hewlett-Packard HP 1100 with PC supported analytical software ChemStation).
[0144] Stationary Phase C18 - Inverted Phase, 5µm, 250 x 4.0mm
[0145] Mobile Phase Milli-Q-Water
[0146] Injection volume 20μl
[0147] Post-column derivatization of acetylacetone solution
[0148] Flow 1.0ml / min
[0149] Detection UV-detection wavelength 420nm
[0150] Sample preparation Dissolve 250mg in 3ml THF, then top up to 25ml with water
[0151] Calibration solution 100mg formaldehyde solution / 100ml water
[...
Embodiment A
[0202] Example A: Reproduction (Nachstellung) of Example 2 described in DE 892 974
[0203] After adding 240 g of 50% potassium hydroxide solution and 400 g of methanol, 1000 g of 30% formaldehyde solution was added to 1200 g of acetophenone with vigorous stirring within 2 hours. At this point the temperature will rise to 90°C. This temperature was maintained for 10 hours. After acidification with sulfuric acid, the resulting coagulum is washed with hot water, melted and removed in vacuo.
[0204] 1260 g of yellow resin were obtained. The resin is transparent and brittle, and has a melting point of 67°C. The Gardner color number was 3.8 (50% in ethyl acetate). For example, it is soluble in acetates such as butyl acetate and ethyl acetate, and in aromatic compounds such as toluene and xylene. Insoluble in ethanol. The formaldehyde content was 255ppm.
Embodiment B
[0205] Example B: Reproduction of Example 3 described in DE 33 34 631 A1
[0206] The resin obtained according to Example A was hydrogenated continuously in a trickle-bed reactor at 300 bar and a temperature of 180° C. according to Example 3 described in DE 33 34 631 A1. 100 mL of Harshaw-Ni-5124 catalyst (available through Engelhard Corporation) was added to the reactor. 50 mL of 30% resin solution was added to isobutanol every hour to replenish the consumed hydrogen so that the pressure in the reactor was kept constant at 300 bar.
[0207] Embodiments of the Invention
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com