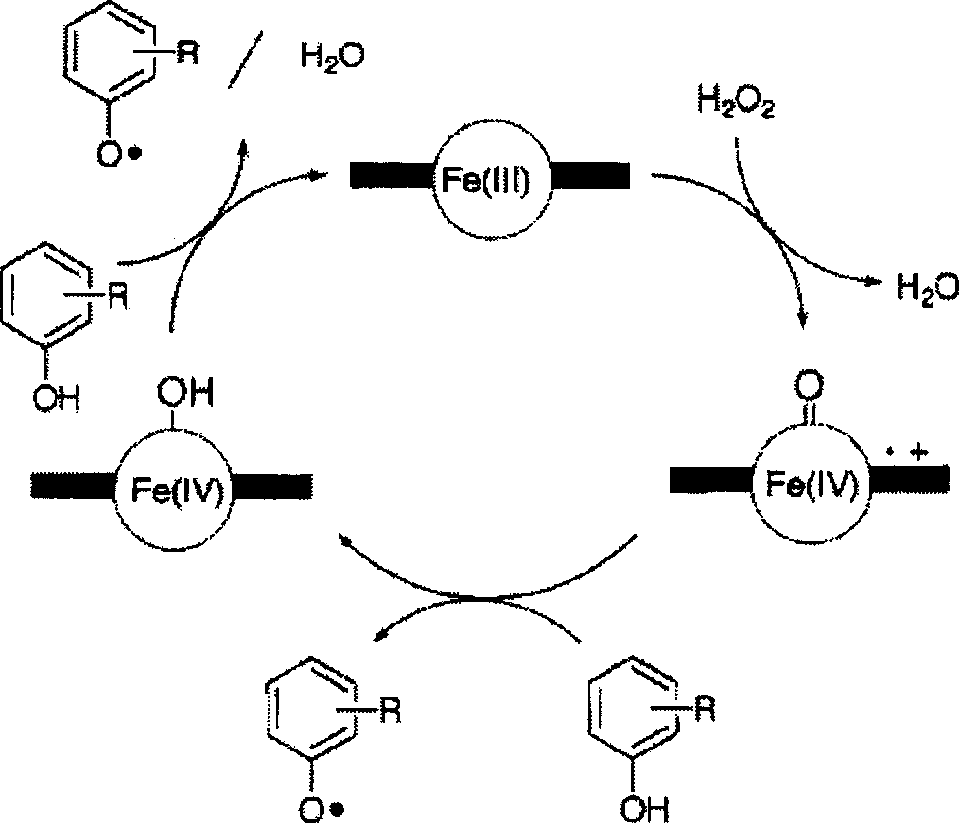
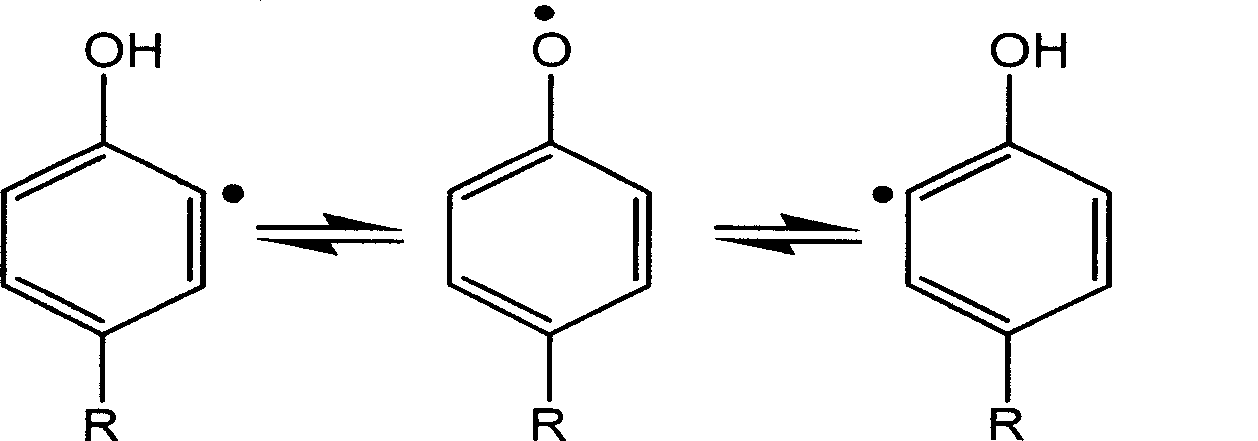
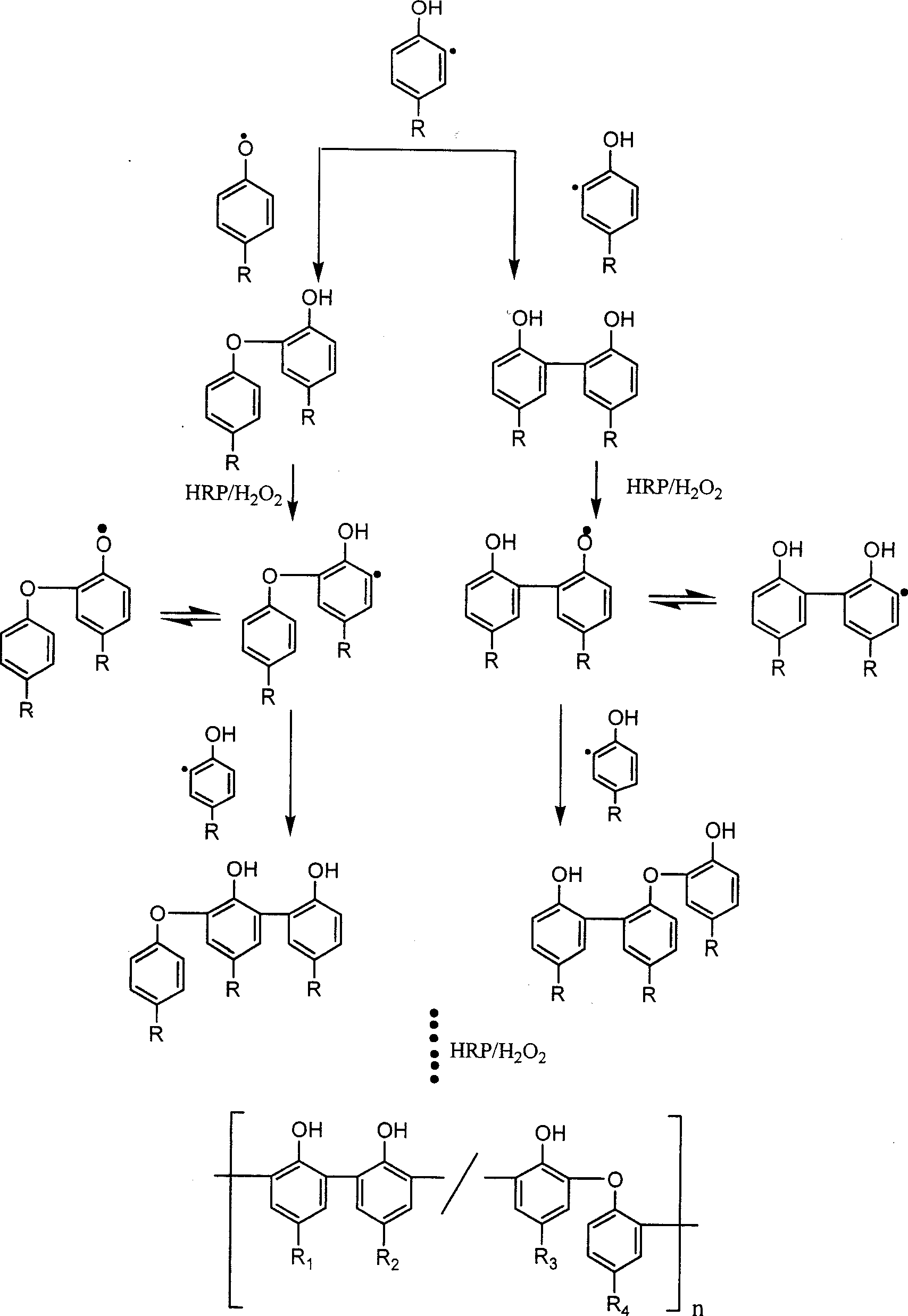
Preparation of enzyme-catalyzed rapid-solidified hydrogel and use thereof
A rapid curing and hydrogel technology, applied in the field of medical biomaterials, can solve the problems of cumbersome preparation steps, unsatisfactory hydrogel mechanical strength, and difficulty in controlling the substitution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Synthesis of Difunctional Polyethylene Glycol Monomer Substituted by p-Hydroxyphenylpropionic Acid
[0064]20 g of difunctional polyethylene glycol (Mn=4000, 5 mmol), 3.32 g of p-hydroxyphenylpropionic acid (20 mmol) and 400 mg of p-toluenesulfonic acid were added to a 25 ml one-necked flask at one time, and then 60 ml of toluene was added. Connect the water separator to the flask, connect the spherical condenser to the water separator, and pass the nitrogen protection at the upper end of the condenser. Under rapid magnetic stirring, the temperature of the reaction system is raised to 110-150°C, and the toluene is continuously refluxed to bring out the reaction by-product water, which settles to the lower layer in the water separator. The reaction is generally 4 to 12 hours. After the reaction was complete, the reaction product was precipitated with excess ether. Then adopt the dissolution-precipitation method, dichloromethane as the solvent and anhydrous ether as the...
Embodiment 2
[0066] Synthesis of Tetrafunctional Polyethylene Glycol Monomer Substituted by p-Hydroxyphenylpropionic Acid
[0067] Similar to the method in Example 1. Add 18g of tetrafunctional polyethylene glycol (Mn=36000, 0.5mmol), and 2656g of p-hydroxyphenylpropionic acid (16mmol) and 200mg of p-toluenesulfonic acid into a 250ml single-necked flask at one time, then add 60ml of toluene, and add magnetic force stir bar. Connect a water separator to the single-necked flask, and then connect a spherical condenser to the water separator, and the upper end of the condenser is protected by nitrogen gas. Under rapid magnetic stirring, the temperature of the reaction system is raised to 110-150°C, and the toluene is continuously refluxed to bring out the reaction by-product water, which settles to the lower layer in the water separator. The reaction is generally more than 4 to 12 hours. After the reaction was complete, the reaction product was precipitated with ether. And adopt the dissol...
Embodiment 3
[0069] Synthesis of Polyethylene Glycol-Polypropylene Glycol-Polyethylene Glycol Copolymer (Pluronic F127) Substituted by P-Hydroxyphenylpropionic Acid
[0070] Similar to the method in Example 1. 12.6g of Pluronic F127 (Mn=12600, 1mmol), 1.328g of p-hydroxyphenylpropionic acid (8mmol) and 160mg of p-toluenesulfonic acid were added to a 250ml one-necked flask at one time, and then poured into 60ml of toluene. The single-necked flask is connected as a water separator, and a spherical condenser is connected to the water separator, and the upper end of the condenser is protected by nitrogen gas. After the device is connected, under rapid magnetic stirring, the temperature of the reaction system is raised to 110-150°C, allowing the toluene to generate continuous reflux, taking out the reaction by-product water, and the water settles to the lower layer in the water separator. The reaction is generally more than 4 to 12 hours. After the reaction was completed, the obtained toluene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com