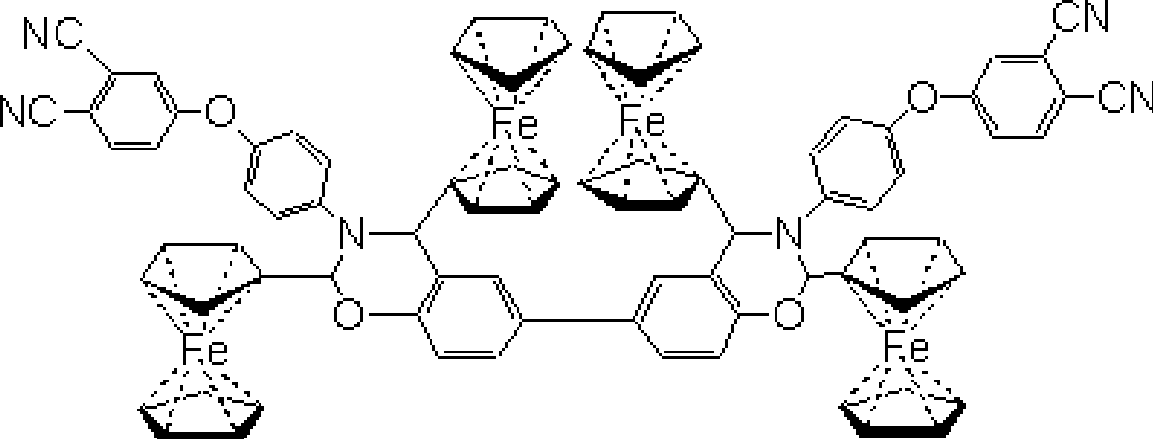
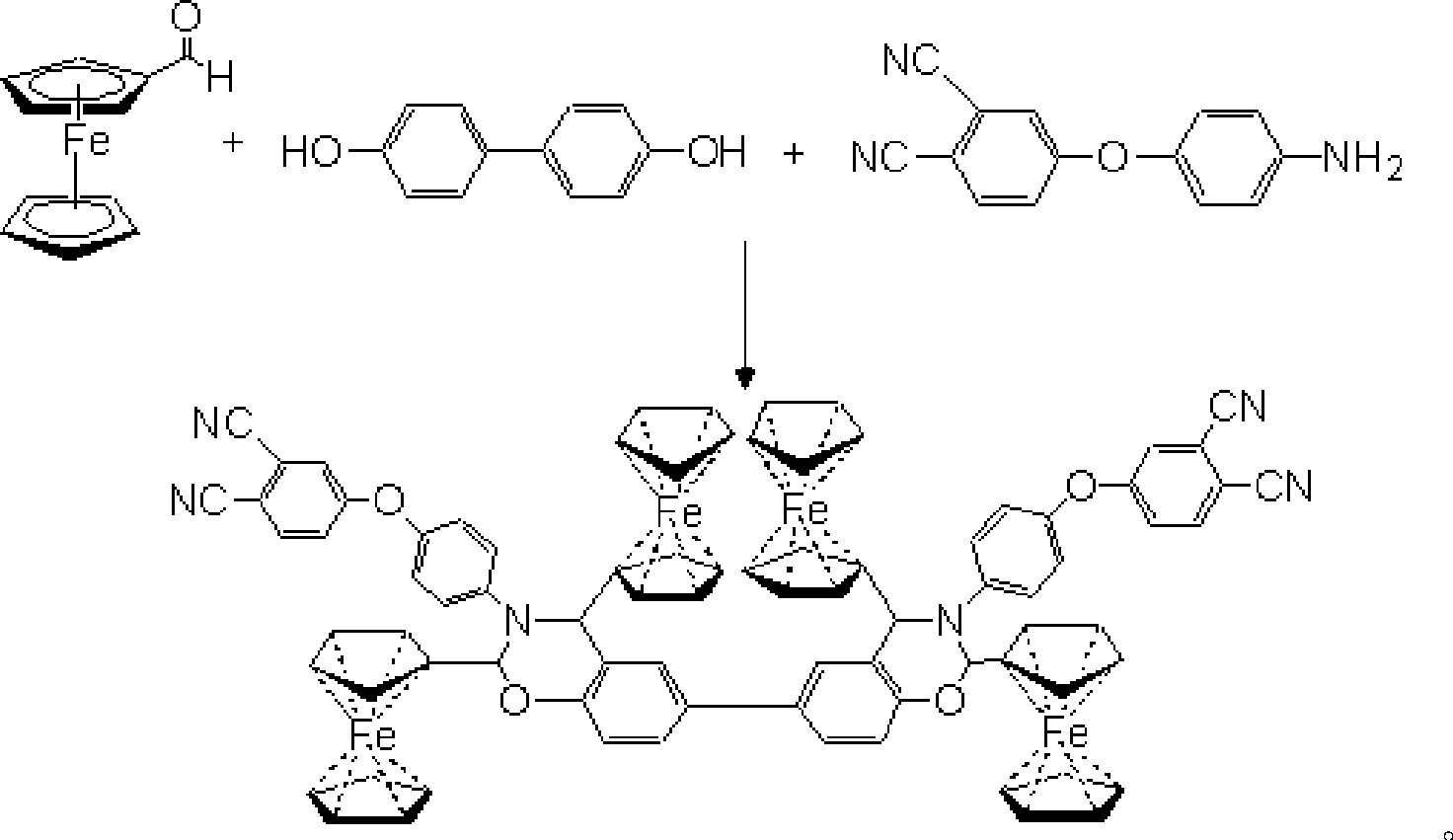
Magnetic ferrocene-double-terminal phthalonitrile resin, condensate and preparation thereof
A kind of base phthalonitrile resin, the technology of base phthalonitrile, applied in cured product and their preparation, the field of magnetic ferrocene-double-end phthalonitrile resin, can solve the problem of synthetic magnetic ferrocene - double-end phthalonitrile resin and other problems, to achieve the effects of controllable reaction process and structure, good magnetic properties, simple and effective method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Preparation of magnetic ferrocene-two-terminal phthalonitrile resin
[0035] Raw material consumption is (by mol):
[0036] Ferrocene formaldehyde 4
[0037] Biphenol 1
[0038] 4-(4-Aminophenoxy)phthalonitrile 2
[0039] N,N-Dimethylformamide 15
[0040] Ferrocene formaldehyde, 4-(4-aminophenoxy) phthalonitrile, biphenol and N, N-dimethylformamide are added in the four-necked bottle according to the above-mentioned mol ratio, and passed into Nitrogen was used to replace the air in it, and the temperature was raised to 90-100° C. for 6 hours, and nitrogen was used to protect the whole reaction process. Pour the reaction mixture into 0.1M / L NaOH solution to precipitate, filter, and wash the obtained solid with ethanol several times, then place it in a vacuum oven and dry it at 60°C for 24 hours to obtain the magnetic ferrocene-double-terminal phthalate Dicyanonitrile resin, yield 73%, curing peak temperature is double curing peak, respectively 221 ° C and 262.2 ...
Embodiment 2
[0051] The preparation method is the same as in Example 1, except that the iron trichloride in the step (2) is changed to ferric nitrate, and the properties of the magnetic ferrocene-two-end phthalonitrile resin prepared are as follows: yield 74.1%, The curing peak temperature is a double curing peak, respectively 221.3°C and 263.2°C, and the FTIR spectrum shows a 945cm -1 The characteristic absorption peak of the benzoxazine ring, 2225cm -1 The characteristic absorption peak of the nitrile group.
[0052] The properties of the cured resin obtained are as follows: the saturation magnetization of the cured product is 1.95575emu / g, the residual magnetism reaches 0.12012emu / g, and the coercive force is 73.310e. The Curie temperature is above 500K. The glass transition temperature is 313°C; the decomposition temperature (5%) is 422°C; and the carbon residue rate in nitrogen atmosphere at 800°C is 71.4%.
Embodiment 3
[0054]The preparation method is the same as in Example 1, except that N, N-dimethylformamide in step (1) is replaced with N-methylpyrrolidone, and the magnetic ferrocene-two-end phthalonitrile resin obtained has the following properties : Yield 74.7%, DSC curing peak temperature is double curing peak, respectively 222.3 ℃ and 264.2 ℃, FTIR spectrum shows 945cm -1 The benzoxazine ring characteristic, 2225cm -1 There are characteristic absorptions of nitrile groups.
[0055] The properties of the cured resin obtained are as follows: the saturation magnetization of the cured product is 1.98375emu / g, the residual magnetism reaches 0.12852emu / g, and the coercive force is 74.210e. The Curie temperature is above 500K. The glass transition temperature is 315°C; the decomposition temperature (5%) is 424°C; the carbon residue rate in nitrogen atmosphere at 800°C is 72.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com