Epidermal growth factor nano lipid vector preparation for skin and preparation method thereof
A technology of epidermal growth factor and nano-lipid carrier, which is applied in liposome delivery, skin diseases, medical preparations containing active ingredients, etc. It can solve the problems of poor stability of hEGF, low absorption and utilization of active ingredients, and transdermal effect of preparations Insufficient and other problems, to achieve good physiological compatibility, improve placement stability, good skin moisturizing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
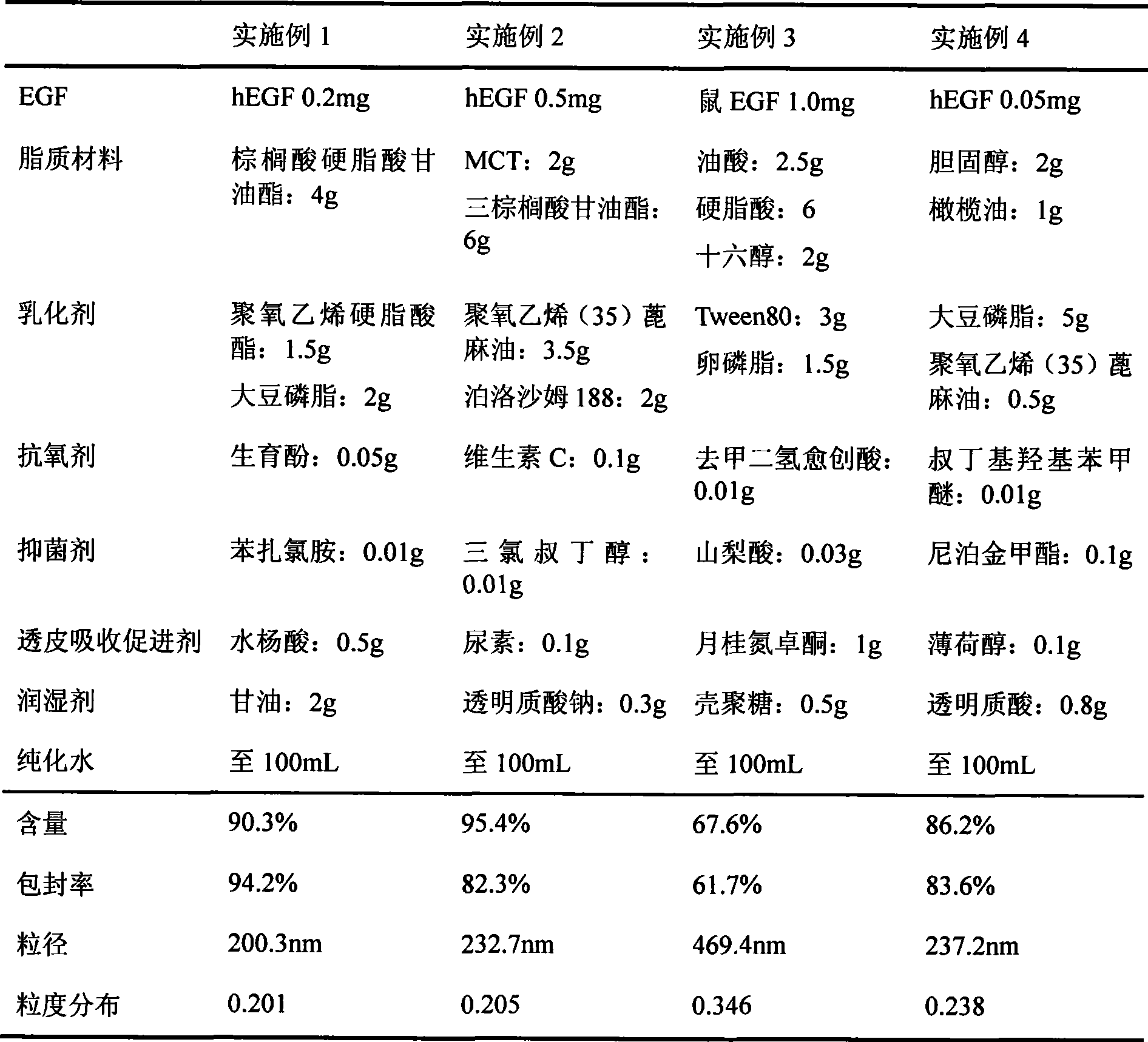
[0050] Table 1 shows the ingredients and contents of the epidermal growth factor nano-lipid carrier preparations for skin in Examples 1-4, and also shows the encapsulation efficiency and particle size measurement results of the preparations.
[0051] Table 1 Components and content, encapsulation efficiency, particle size determination results of the preparations of Examples 1-4
[0052]
[0053] ① Preparation of hEGF nano-lipid carrier:
[0054] According to the composition of Example 1 given in Table 1, 0.2 mg of hEGF was weighed and dissolved in 500 μL of pure water to obtain water phase 1; 1.5 g of polyoxyethylene stearate (S-40) and 2 g of glycerin were dissolved in the remaining Measure the pure water to obtain the water phase 2; the lipid material glyceryl palmitostearate 4g, soybean lecithin 2g, and tocopherol 0.05g are dissolved in 3mL of dichloromethane to obtain the oil phase; Slowly add water phase 1 and water phase 2 into the oil phase, stir for 1 min to form col...
Embodiment 2
[0066] According to the composition of Example 2 given in Table 1, weigh 0.5 mg of hEGF and dissolve it in 500 μL of pure water to obtain the water phase 1; dissolve 0.3 g of sodium hyaluronate and 2 g of poloxamer 188 in the remaining amount of pure water , to obtain the water phase 2; the lipid material tripalmitin 6g, MCT 2g, polyoxyethylene (35) castor oil 3.5g were dissolved in 3mL of dichloromethane to obtain the oil phase; under high shear conditions, the water Slowly add phase 1 and water phase 2 into the oil phase, stir for 1 min to form colostrum; place the colostrum in a rotary evaporator, and remove dichloromethane under reduced pressure at 30°C; then, use a homogenizer to homogenize at a pressure of 500 bar, Add 0.01 g of chlorobutanol, 0.1 g of urea, and 0.1 g of vitamin C to obtain hEGF nano lipid carrier preparation for skin.
[0067] The determination results of the content, encapsulation efficiency, average particle size and particle size distribution of the ...
Embodiment 3
[0070] According to the composition of Example 3 given in Table 1, 1.0 mg of mouse EGF was weighed and dissolved in 500 μL of pure water to obtain an aqueous phase 1; chitosan was dissolved in the remainder water containing 1.5% acetic acid to obtain an aqueous phase 2; Dissolve 2.5g of lipid materials oleic acid, 6g of stearic acid, 2g of cetyl alcohol, 3g of Tween80, 1.5g of lecithin, and 1g of laurocaprazine in 3mL of dichloromethane to obtain an oil phase; Slowly add water phase 1 and water phase 2 into the oil phase, stir for 1 min to form colostrum; put the colostrum in a rotary evaporator, remove dichloromethane under reduced pressure at 30°C; then carry out high-pressure homogenization, and add nordihydro Guaiac acid and sorbic acid are used to obtain a mouse EGF nano lipid carrier preparation for skin; and then spray-dried to obtain a spray-dried powder.
[0071] Table 1 shows the content, encapsulation efficiency, average particle size and particle size distribution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com