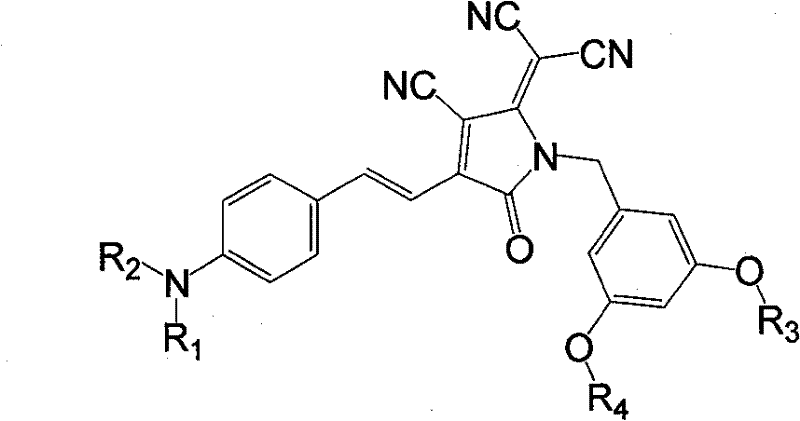
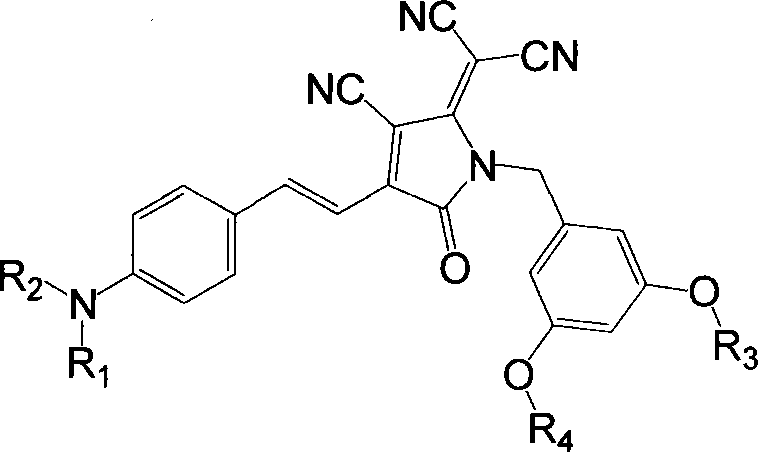
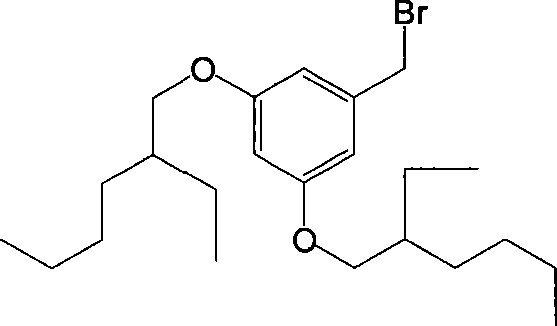
Organic second order non-linear optical chromophore group containing dendritic structured tricyano pyrroline receptor, synthesizing method and use thereof
A tricyanopyrroline and second-order nonlinear technology, which is applied in the fields of organic chemistry, chemical instruments and methods, and luminescent materials, can solve the problems of not being able to meet the needs of deviceization, low polymer solubility, and low polarization efficiency, etc. problems, to achieve the effects of improving polarization efficiency, reducing synthesis steps, improving solubility and film-forming properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of 3,5-Diisooctyloxybenzyl Bromide
[0033]
[0034] 2.9 grams (0.021mol) of 3,5-dihydroxybenzyl alcohol and 9.6 grams (0.050mol) of bromoisoctane were dissolved in 50 ml of DMF, and 10 g of anhydrous potassium carbonate was added under stirring, and the reaction was carried out at 75°C for 24 hours. Most of the DMF was distilled off under reduced pressure, an appropriate amount of saturated aqueous sodium chloride was added, extracted with ether, dried over anhydrous magnesium sulfate, the desiccant was removed by filtration, and the ether was removed by rotary evaporation to obtain an orange-yellow oily liquid, which was separated by silica gel column chromatography to obtain 3,5- 5.4 grams of diisooctyloxybenzyl alcohol, yield 71%.
[0035] Dissolve 1.0 g (0.0027 mol) of 3,5-dioctyloxybenzyl alcohol with a small amount of anhydrous THF (about 20 ml), and add 1.1 g (0.0034 mol) of tetrabromide dropwise to the solution under nitrogen protection. carbonize...
Embodiment 2
[0038] Synthesis of Tricyanopyrroline (TCP) Acceptor
[0039]
[0040] Dissolve 2.32 g (20 mmol) of ethyl pyruvate in 10 ml of ethanol, and then add 1.32 g (10 mmol) of malononitrile dimer. The mixture was refluxed for 1 hour under the protection of nitrogen, and the ethanol was removed by rotary evaporation, dissolved and filtered with 50 ml of dichloromethane to obtain a part of insoluble matter, and the filtrate was concentrated to precipitate crystals. In order to obtain the pure product, it can be separated by silica gel column chromatography, and the yield is about 13%.
Embodiment 3
[0042] Synthesis of Chromophores Containing TCP Acceptors
[0043]
PUM
| Property | Measurement | Unit |
|---|---|---|
| electro-optic coefficient | aaaaa | aaaaa |
| electro-optic coefficient | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com