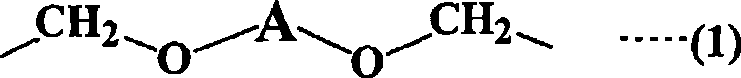
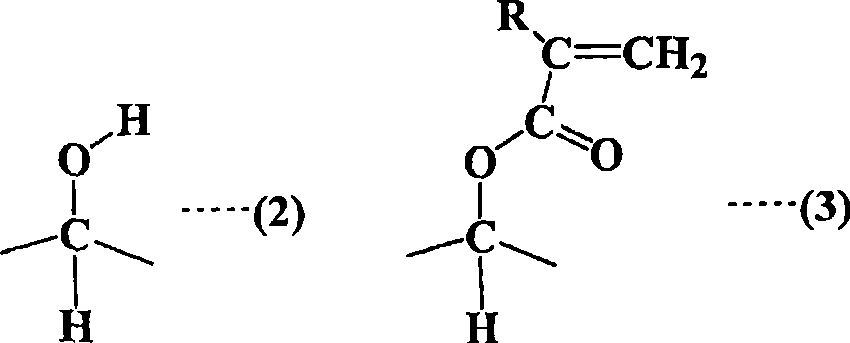
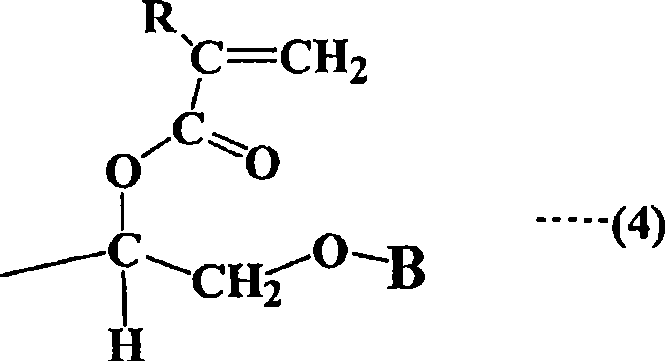
Straight-chain compound containing (methyl) acryloyl, star-shaped compound containing (methyl) acryloyl and method for manufacturing them
An acryl group and a manufacturing method technology, applied in the field of acryl group-containing compounds, can solve problems such as poor curability or poor curing sensitivity, and achieve the effects of excellent sensitivity, high curability and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0129]Hereinafter, the present invention will be described in more detail through examples. However, the present invention is not limited to each of the following examples, and for example, components of these examples can be appropriately combined with each other. In addition, "%" means "mass %" unless otherwise specified.
Synthetic example 1
[0130] Synthesis example 1 (synthesis of catalyst tristannoxane compound)
[0131] 16.48 g (75 mmol) of dimethyl tin dichloride, 120 ml of water, and 45 ml of ethanol were added to a reaction vessel equipped with a stirrer, a thermometer, and a dropping funnel, and stirred to dissolve evenly. Then, 10.1 g (100 mmol) of triethylamine was dripped from the dropping funnel while stirring. At this time, the reaction vessel was placed in an ice bath to keep the temperature of the contents at about 20°C. After completion of the dropwise addition, the mixture was further reacted with stirring at the same temperature for 3 hours. After the reaction, the white precipitate was filtered, washed with 100 ml of water and then 200 ml of ethanol, and then dried under reduced pressure at 105° C. to obtain 12.6 g of white powder. As a result of elemental analysis (using vario EL manufactured by elementar company), tin is 64.9%, chlorine is 13.0%, and the theoretical value of hexamethyl-1,5-di...
Embodiment 1
[0134] The transesterification reaction (synthesis of acrylate) of embodiment 1 bisphenol A type epoxy resin
[0135] Add bisphenol A type epoxy resin EPICLON AM-040-P (manufactured by Dainippon Ink Chemical Industry Co., Ltd., number average molecular weight Mn=2000, according to GPC) Polystyrene equivalent value) 10.0 g, ethyl acrylate 120.0 g, tristannoxane compound 0.6 g obtained in the above synthesis example as a catalyst, 1.2 g of p-methoxyphenol as a polymerization inhibitor, while introducing air start reacting. The reaction was carried out for 24 hours while ethanol generated at a reaction temperature of 95 to 98° C. was refluxed and removed as a mixed solution with ethyl acrylate.
[0136] After the reaction, excess ethyl acrylate was removed by vacuum distillation, and a small amount of toluene was added to the residue, and then excess ethyl acrylate was removed by vacuum distillation again.
[0137] The reaction mixture remaining after distillation under reduced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com