Cholagogic pain-relieving capsule as well as preparation method and quality test and control method thereof
A pain-relieving capsule and choleretic technology, applied in the field of drugs for cholecystitis and the treatment of acute and chronic hepatitis, can solve the problems of drug selectivity affecting compliance, long heating time of extract, affecting drug absorption, etc. Reliable curative effect and the effect of improving drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
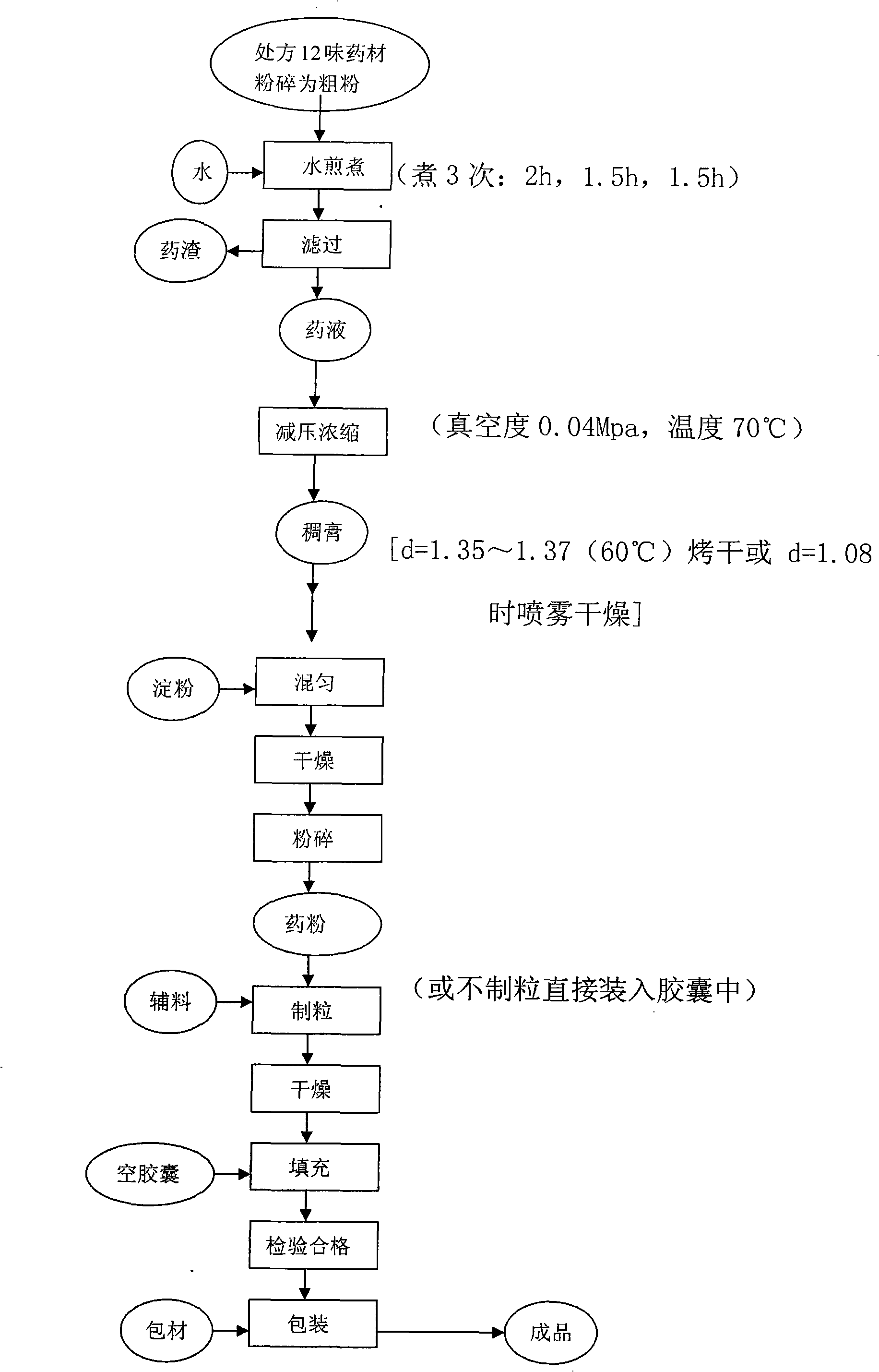
[0041] The raw material medicine that makes capsule active ingredient of the present invention consists of: Bupleurum (stir-fry) 120g, red peony root 120g, Fructus aurantii (stir-fry) 120g, Radix Glycyrrhizae 60g Capillary root 200g, Corydalis Corydalis (stir-fry) 200g, Atractylodes 120g, Toosendan ( Fried) 200g, Agrimony 300g, Banlangen 200g, Dandelion 300g and Turmeric 200g. Crush it into a coarse powder, add water and decoct three times, the first time for 2 hours, the second time and the third time each for 1.5 hours, combine the decoction, filter, and concentrate the filtrate under reduced pressure to a thick liquid with a relative density of 1.35-1.37 (60°C). Paste, add appropriate amount of auxiliary material starch, dry, crush, add appropriate amount of starch and magnesium stearate, mix well, put into capsules; Finally, add an appropriate amount of magnesium stearate, mix evenly, and pack into capsules to make 1000 capsules to obtain the required choleretic pain relie...
Embodiment 2
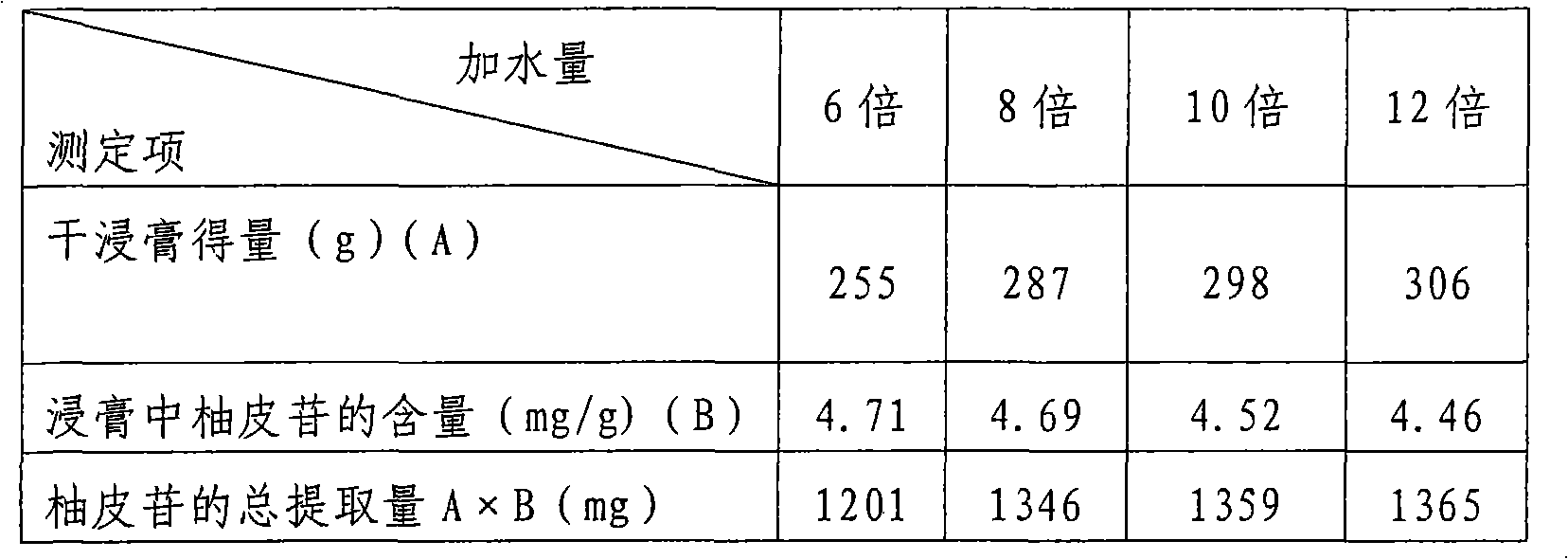
[0043] The optimization method of the water decoction process is: take the prescription medicinal material amount (2140g) that makes 1000 capsules, add water in multiples according to the table, the number of times (three times) of each decoction, and the time (2 hours, 1.5 hours, 1.5 hours) The same, and adjust the water content of each obtained dry extract to be almost the same, the test data are shown in Table 1.
[0044] Table 1.
[0045]
[0046] The method for determining the content of naringin in Table 1 is the HPLC method. Table 1 shows that, taking the total solids as an indicator, the more water is added, the more dry paste will be obtained. For example, taking the final total extraction amount of naringin as an indicator, adding 8 to 10 times the amount of water is enough, and 8 times , 10 times and 12 times the water extraction results are not much different.
Embodiment 3
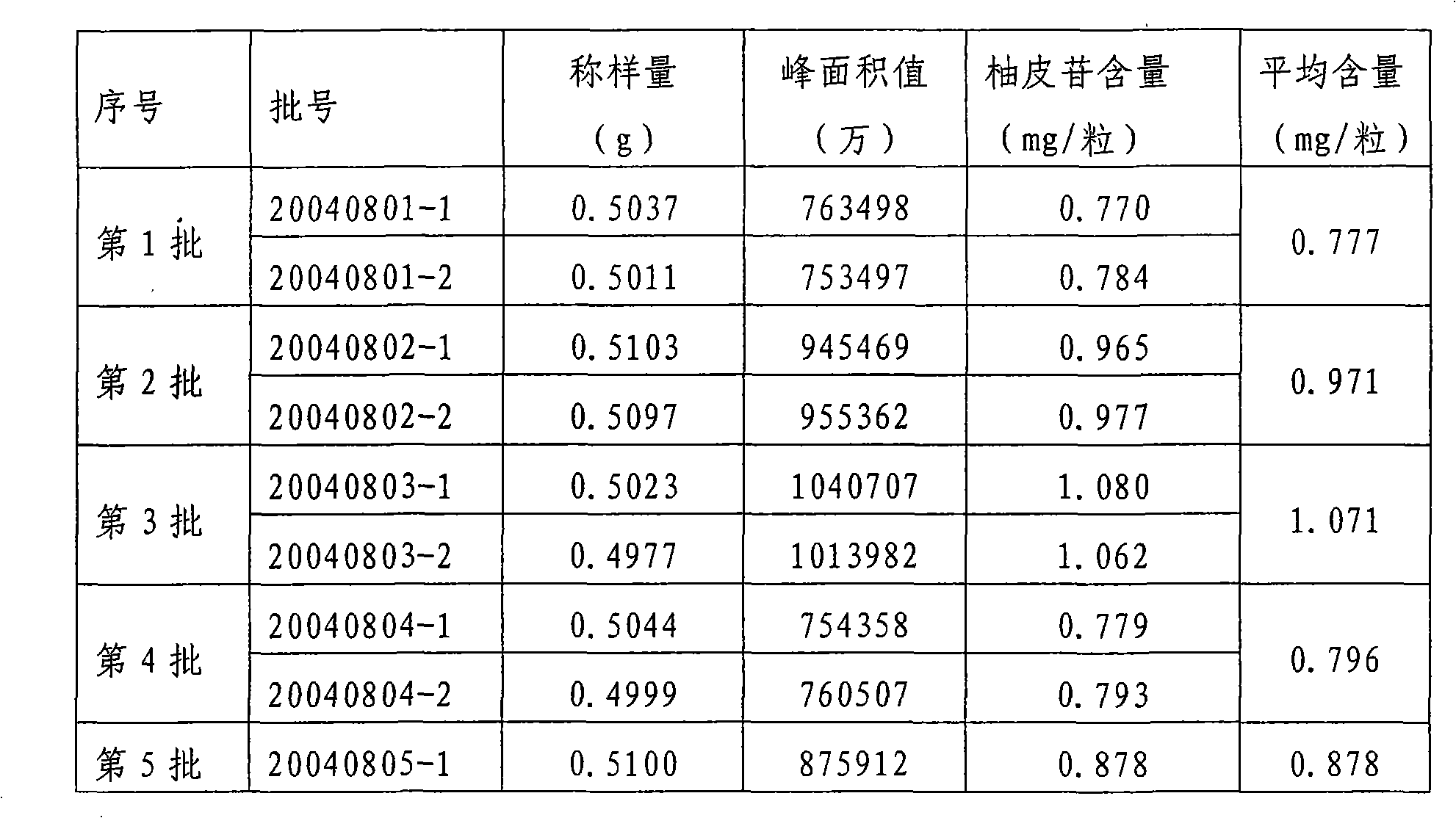
[0048] ——Test data of three batches of pilot production
[0049] *Amplify 10 times according to the prescription quantity, and produce three batches of products according to the above-mentioned manufacturing method, and the test data are shown in the table.
[0050] batch number
[0051] Relative density of extract (80℃)
[0052] The above three batches of finished products have been inspected: properties, identification, water content, disintegration time limit, weight difference, content determination and microbial inspection are all qualified (according to the self-made quality standard draft and the relevant provisions of the appendix of "Chinese Pharmacopoeia").
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com