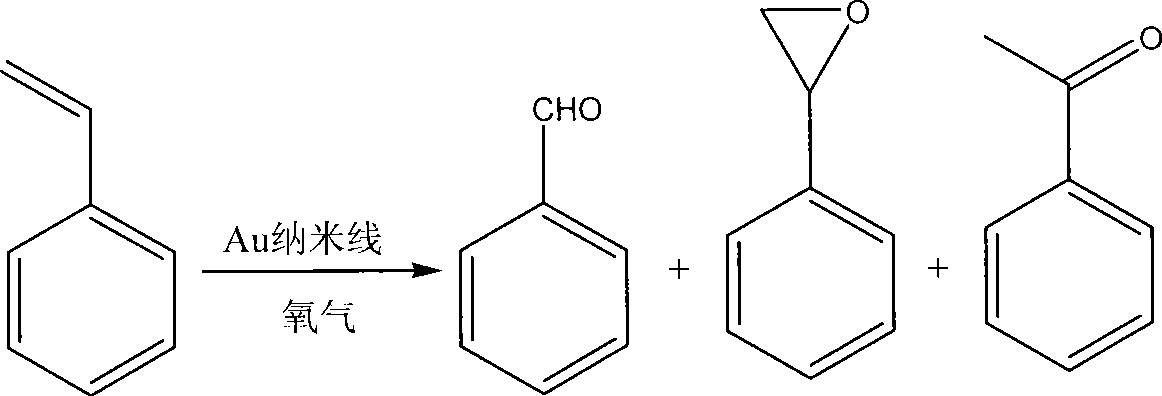
Method for preparing benzaldehyde through styrene catalytic oxidation
A technology for catalytic oxidation and styrene, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds. Effect of high conversion rate and yield improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In the reaction flask, 2.5 g of styrene, 0.5 g of gold nanowire toluene solution (wherein, about 0.5 mg of gold nanowire, diameter less than or equal to 5 nm), and 14.5 g of toluene are loaded into the reaction flask; the system is connected with an oxygen bag and a condenser tube, Then cool down-vacuumize-release oxygen, cycle 3-4 times, put in oxygen, and return to room temperature; the system is heated in an oil bath at 100°C for 24 hours to obtain the product.
[0022] Get product and carry out gas-mass coupling (GC-MS) and gas chromatography (GC) analysis, the result is as follows: transformation rate: 54.3%; Benzaldehyde, benzo epoxy, the selectivity of acetophenone are respectively 51.6%, 43.1%; % and 5.3%, the yield of benzaldehyde is 28.0%.
Embodiment 2
[0024] In the reaction flask, 2.5 g of styrene, 0.8 g of gold nanowire toluene solution (wherein, about 0.8 mg of gold nanowire and a diameter less than or equal to 5 nm), and 14.2 g of toluene are loaded into the reaction flask; the system is connected with an oxygen bag and a condenser tube, Then cool down-vacuumize-release oxygen, cycle 3-4 times, put in oxygen, and return to room temperature; the system is heated in an oil bath at 100°C for 25 hours to obtain the product.
[0025] Get product and carry out gas-mass coupling (GC-MS) and gas chromatography (GC) analysis, the result is as follows: transformation rate: 54.7%; Benzaldehyde, benzo epoxy, the selectivity of acetophenone are respectively 52.0%, 44.5%; % and 3.5%, the yield of benzaldehyde is 28.4%.
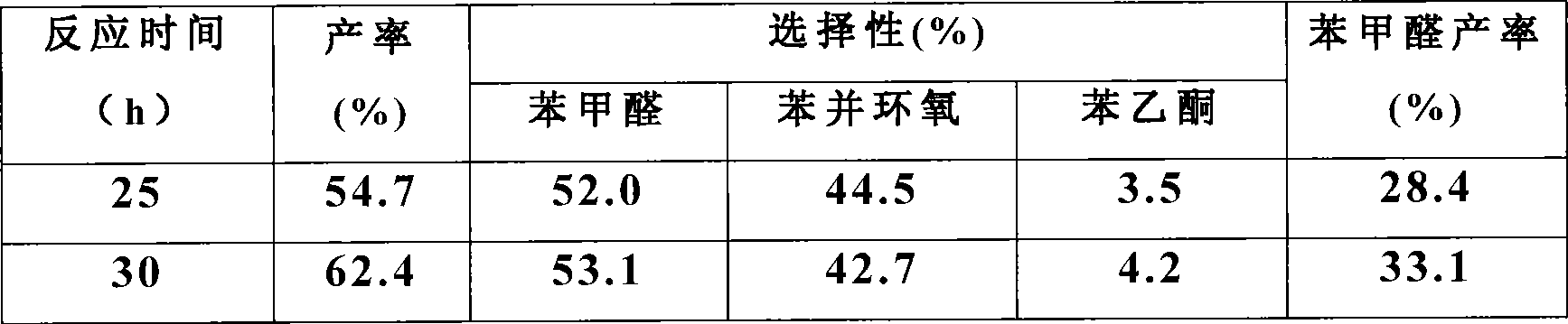
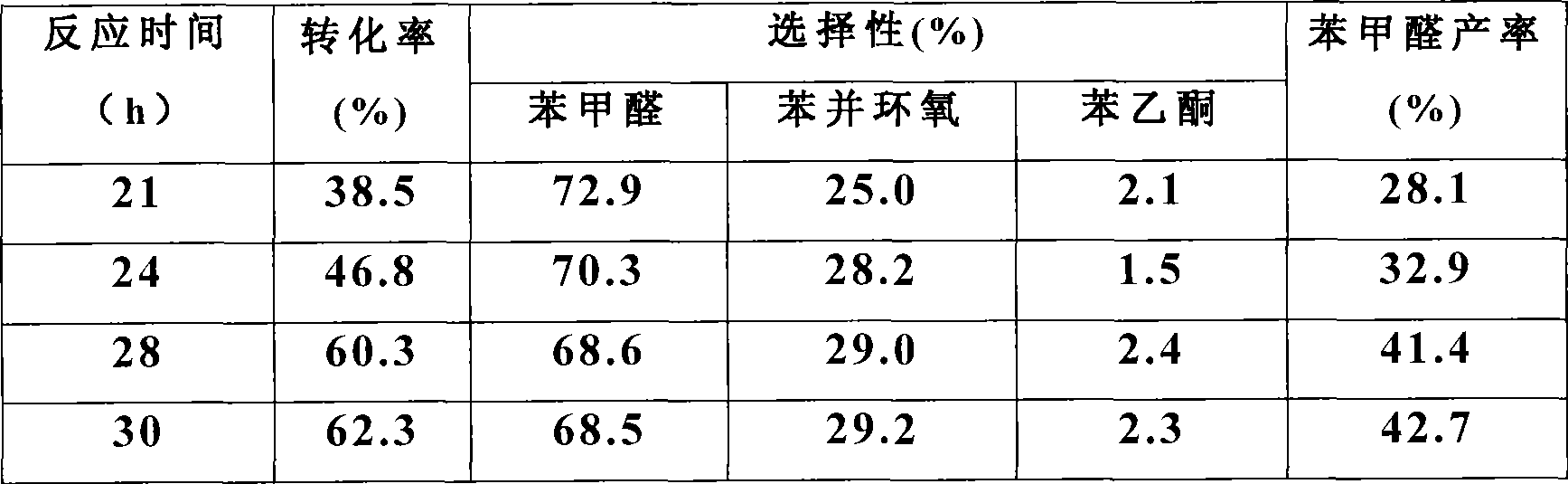
[0026] Measured the impact of reaction time simultaneously, the result is as follows:
[0027]
[0028] It can be seen that with the increase of the reaction time, the conversion rate of styrene and the yield of b...
Embodiment 3
[0030] In the reaction flask, 2.5 g of styrene, 1.2 g of gold nanowire toluene solution (wherein, about 1.2 mg of gold nanowire and a diameter less than or equal to 5 nm), and 13.8 g of toluene are loaded into the reaction flask; the system is connected with an oxygen bag and a condenser tube, Then cool down-vacuumize-release oxygen, cycle 3-4 times, put in oxygen, and return to room temperature; the system is heated in an oil bath at 100°C for 24 hours to obtain the product.
[0031] Get product and carry out gas-mass coupling (GC-MS) and gas chromatography (GC) analysis, the result is as follows: transformation rate: 43.6%; Benzaldehyde, benzo epoxy, the selectivity of acetophenone are respectively 66.1%, 30.8%; % and 3.1%, the yield of benzaldehyde is 28.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com