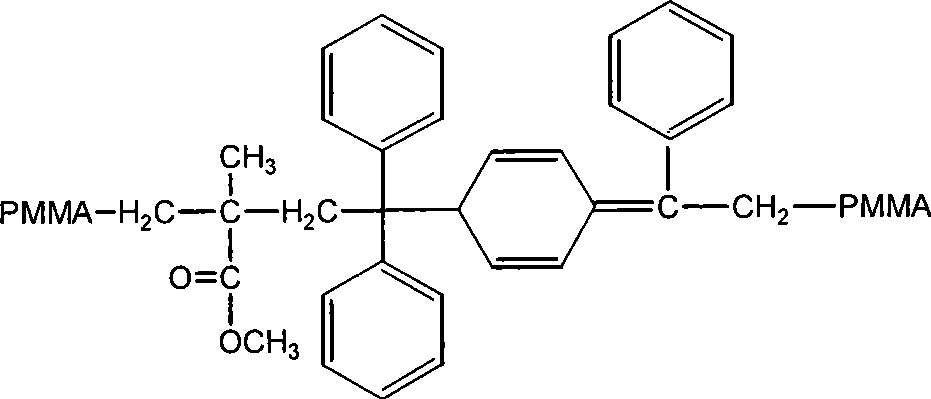
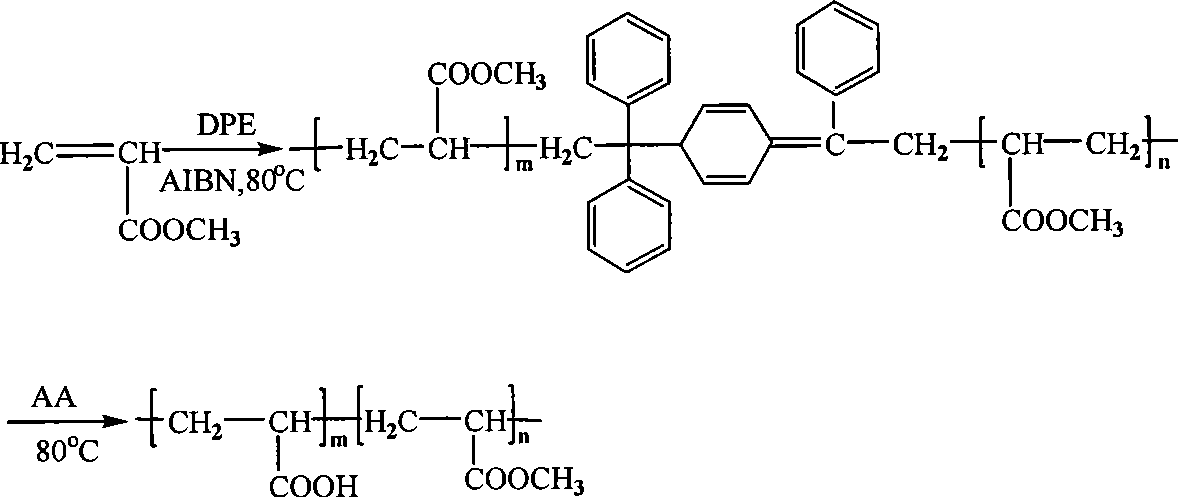Method for preparing methyl methacrylate-acrylic acid block polymer with the existence of 1, 1-diphenylethylene
A technology of methyl methacrylate and diphenylethylene, applied in the field of polymer chemistry, can solve problems such as the preparation of methyl methacrylate-acrylic acid block polymer, which is beneficial to industrial production and avoids hydrolysis. , the effect of simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1) Add 20.0 g of monomer methyl methacrylate (0.2 mol), initiator azobisisobutyronitrile 196.8 mg (1.2 mmol) and 1,1-diphenylethylene 324 mg (1.8 mmol) to In a single-branch tube reaction flask containing a magnetic stirring rotor, after reacting at 70°C for 3 hours, the reaction was stopped and the reaction system was poured into methanol for precipitation, filtered and dried, and the PMMA precursor obtained was re-dissolved with chloroform and precipitated with methanol. To remove the unreacted monomer, initiator and 1,1-diphenylethylene, the obtained polymer powder was placed in a vacuum oven for 24 hours; the conversion rate of the monomer measured by the weighing method was 19%, and the molecular weight and molecular weight distribution of the product obtained : Mn=12900, PDI=1.45;
[0019] 2) Add 0.5g PMMA precursor, 2.5g acrylic acid and 20mL 1,4-dioxane into a single-branch tube reaction flask containing a magnetic stirring rotor, mix well and polymerize acrylic mon...
Embodiment 2
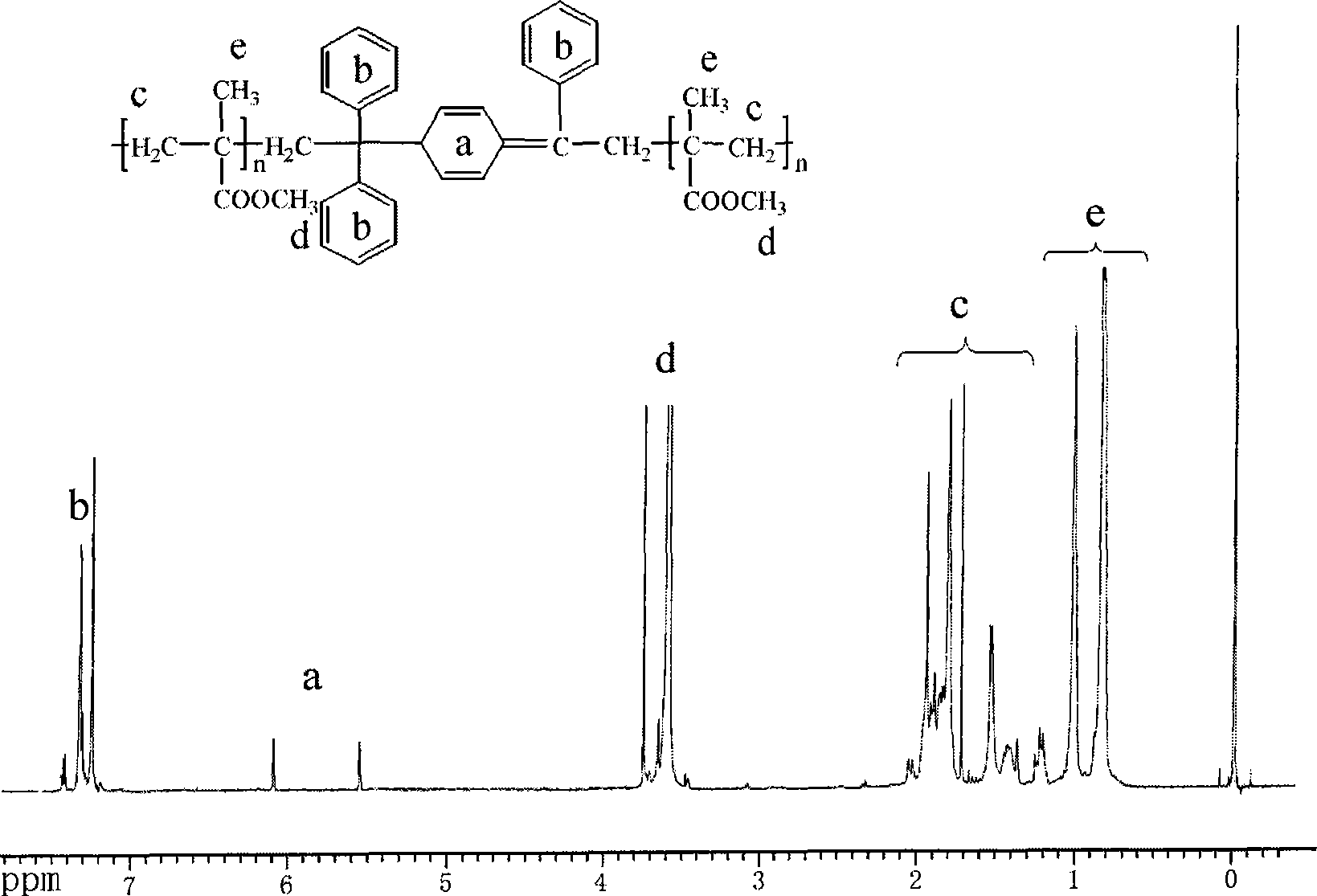
[0022] 1) Add 20.0 g of methyl methacrylate (0.2 mol), 131.2 mg (0.8 mmol) of azobisisobutyronitrile catalyst, and 216 mg (1.2 mmol) of 1,1-diphenylethylene to the magnetic stirring In the single-branch tube reaction flask of the rotor, react at 80℃ for 3h, stop the reaction and pour the reaction system into methanol for precipitation, filter and dry, redissolve the PMMA precursor obtained with chloroform, and precipitate with methanol to remove Unreacted monomer, initiator and 1,1-diphenylethylene, and the resulting polymer powder is placed in a vacuum oven for 24 hours. The conversion rate of the monomer measured by the weighing method is 22%, and the product is 1 The H NMR spectrum is shown as figure 1 As shown, no impurities were seen. The molecular weight and molecular weight distribution were determined by gel permeation chromatography, and narrow-distributed polystyrene was used as a standard sample. The results obtained: Mn=13400, PDI=1.40.
[0023] 2) Add 0.5g PMMA precur...
Embodiment 3
[0026] 1) Add 20.0 g of methyl methacrylate (0.2 mol), 196.8 mg (1.2 mmol) of azobisisobutyronitrile catalyst and 324 mg (1.8 mmol) of 1,1-diphenylethylene to the magnetic stirring In the single-branch tube reaction flask of the rotor, react at 80℃ for 3h, stop the reaction and pour the reaction system into methanol for precipitation, filter and dry, redissolve the PMMA precursor obtained with chloroform, and precipitate with methanol to remove Unreacted monomer, initiator and 1,1-diphenylethylene, and the resulting polymer powder is placed in a vacuum oven for 24 hours. The conversion rate of the monomers measured by the weighing method was 21%, and the molecular weight and molecular weight distribution of the obtained product: Mn = 11700, PDI = 1.36;
[0027] 2) Add 0.5g PMMA precursor, 0.5g acrylic acid and 20mL 1,4-dioxane into a single-tube reaction flask containing a magnetic stirring rotor, mix well and polymerize acrylic monomers under nitrogen protection, the reaction tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com