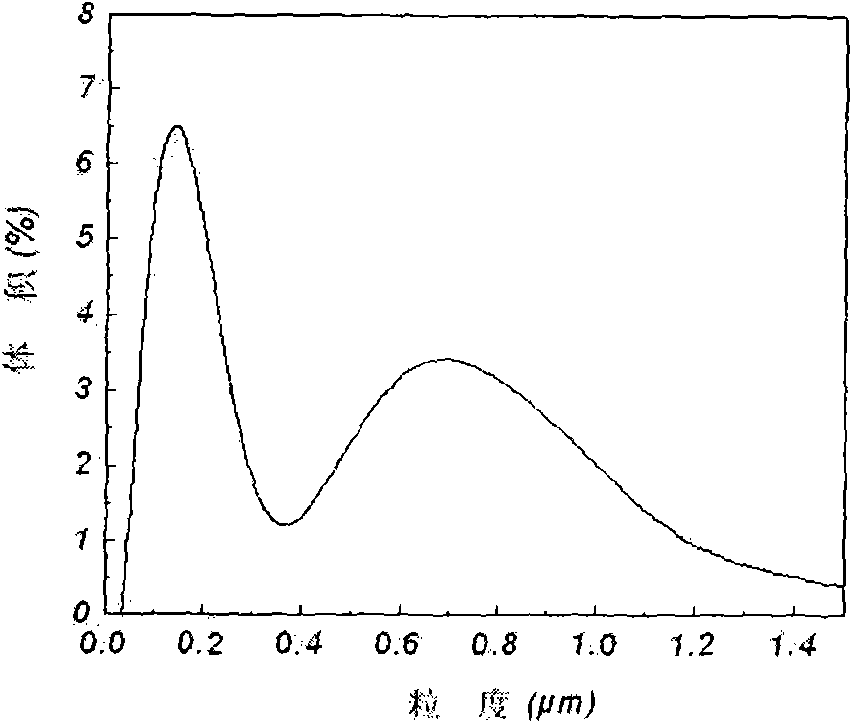
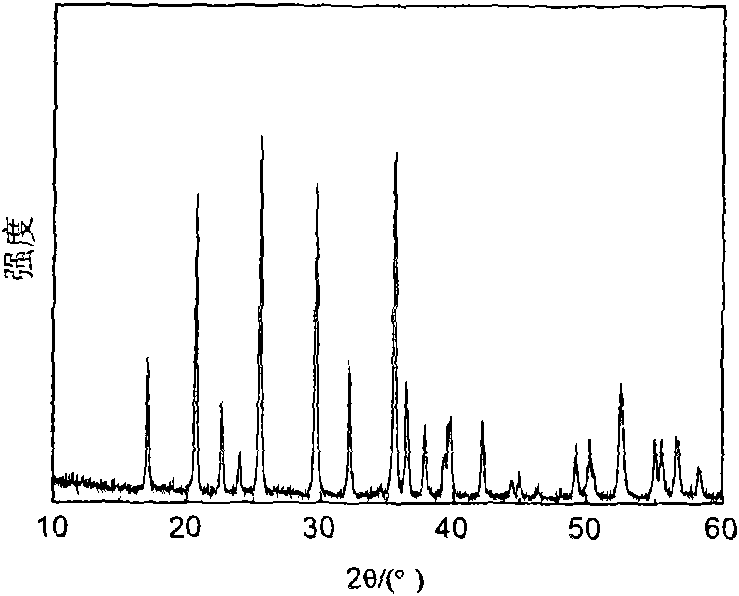
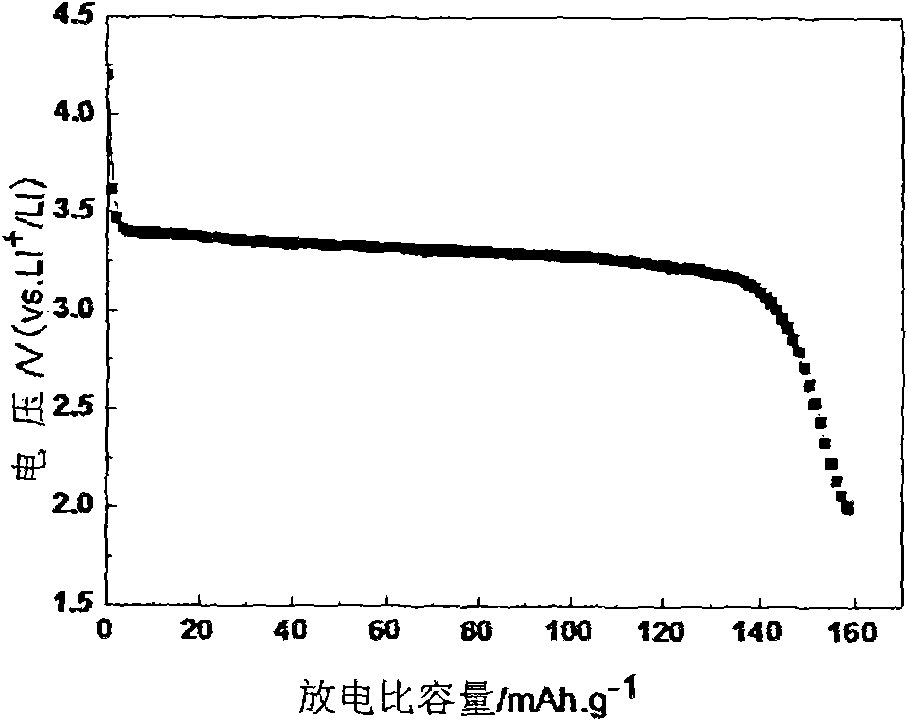
Lithium iron phosphate cathode material and preparation method thereof
A technology for lithium iron phosphate and positive electrode materials, applied in chemical instruments and methods, phosphorus compounds, battery electrodes, etc., can solve the problems of difficult control of metal powder uniform distribution, complicated process, high cost, etc., and achieve strong complementarity between large and small particles, production The effect of simple process and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In the first step, 0.1 mol of lithium phosphate is prepared. First prepare 100ml of 1mol / L ammonium dihydrogen phosphate aqueous solution and 300ml of 1mol / L lithium hydroxide aqueous solution: pour 300ml of 1mol / L lithium hydroxide aqueous solution into a 1L three-necked flask as the base liquid, and add molar ratio of hydrogen Add polyethylene glycol 10000 dispersant to lithium oxide: polyethylene glycol ratio of 1000:1. Preheat the water bath to 60°C, put the three-necked flask in the water bath, control the flow rate of 1mol / L ammonium dihydrogen phosphate aqueous solution and stir at the same time, the average speed flow rate is 50ml / h in the first 60 minutes, and 200ml / h in the last 15 minutes . After the reaction, the frequency was 22KHz, the power was 1000W ultrasonic dispersion for 10 minutes and stirring for 20 minutes respectively, rinsed with distilled water for 5 times, and vacuum dried at 100°C to obtain lithium phosphate.
[0036] In the second step, 0....
Embodiment 2
[0040] In the first step, 0.1 mol of lithium phosphate is prepared. First prepare 100ml of diammonium hydrogen phosphate aqueous solution of 1mol / L, 150ml of lithium carbonate aqueous solution of 1mol / L: pour in the there-necked flask of 1L with the lithium carbonate aqueous solution of 150ml 1mol / L, add lithium carbonate in molar ratio: Add polyethylene glycol 10000 dispersant at a ratio of 1000:1 to polyethylene glycol. Preheat the water bath to 50°C, put the three-necked flask in the water bath, control the flow rate of 1mol / L diammonium hydrogen phosphate solution and stir at the same time, the average speed flow rate is 50ml / h in the first 60 minutes, and 200ml / h in the last 15 minutes . After the reaction was completed, the frequency was 22KHz, the power was 1000W ultrasonic dispersion for 15 minutes and stirring for 15 minutes respectively, rinsed with distilled water for 5 times, and vacuum dried at 100°C to obtain lithium phosphate.
[0041] In the second step, 0.1 ...
Embodiment 3
[0045] In the first step, 0.2 mol of lithium phosphate is prepared. First prepare 100ml of phosphoric acid solution of 2mol / L, 300ml of lithium hydroxide aqueous solution of 2mol / L: pour in the three-necked flask of 1L with the bottom solution of lithium hydroxide aqueous solution of 300ml 2mol / L, add molar ratio lithium hydroxide: poly Add polyethylene glycol 10000 dispersant at 1000:2 ratio of ethylene glycol. Preheat the water bath to 70°C, put the three-neck flask in the water bath, control the flow rate of 2mol / L phosphoric acid solution, the average speed flow rate is 50ml / h in the first 60 minutes, and 200ml / h in the last 15 minutes. After the reaction, the frequency was 22KHz, the power was 1000W ultrasonic dispersion for 10 minutes and stirring for 20 minutes respectively, rinsed with distilled water for 5 times, and vacuum dried at 100°C to obtain lithium phosphate.
[0046]In the second step, 0.2 mol of iron phosphate is prepared. First prepare 100ml of 2mol / L fer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tap density | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com