Rizatriptan benzoate film agent
A technology of rizatriptan benzoate and film preparations, which is applied in medical preparations of non-active ingredients, organic active ingredients, nervous system diseases, etc., to achieve the effects of convenient use, low production cost, and rapid dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
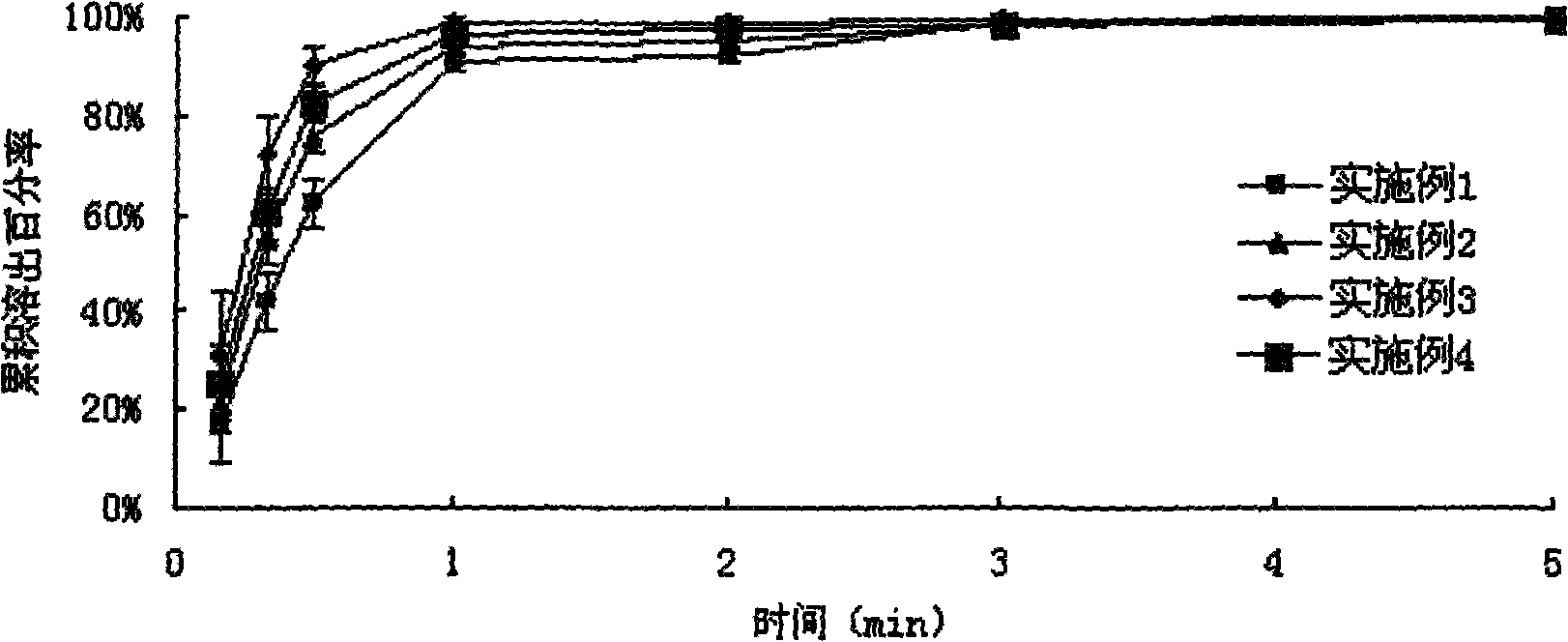
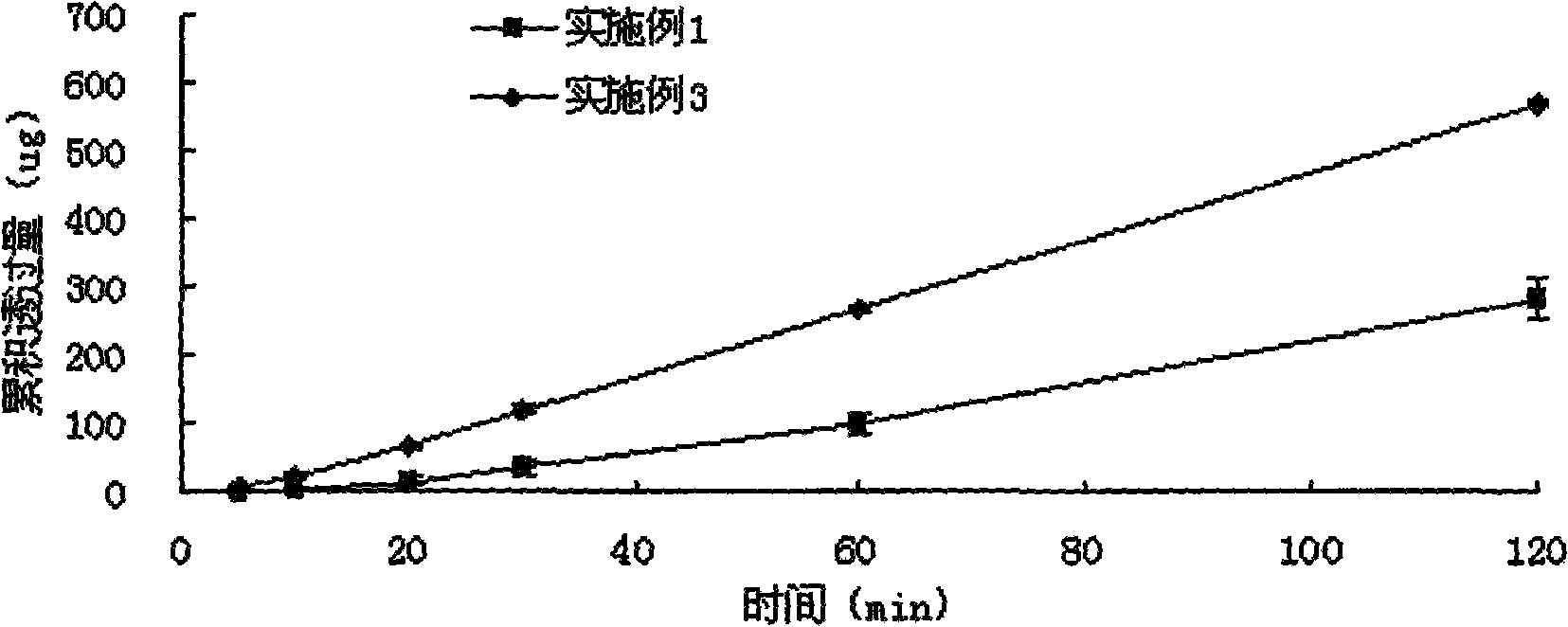
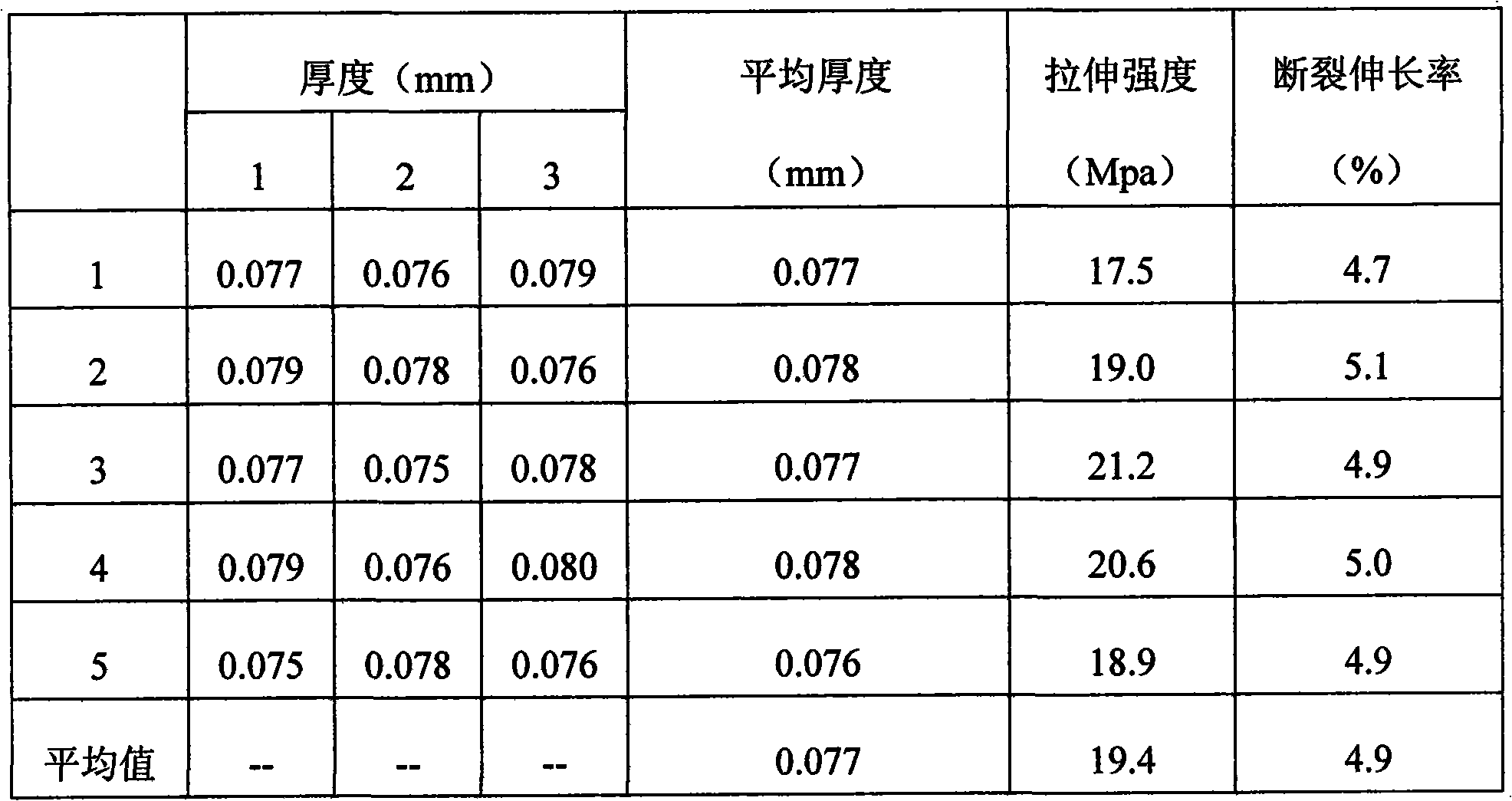
Examples
Embodiment 1
[0062] prescription:
[0063] Rizatriptan Benzoate 10g
[0064] HPMC 56g
[0065] Glycerin 10g
[0066] Tween-80 2g
[0067] Titanium dioxide 2g
[0068] Aspartame 20g
[0069] Preparation:
[0070] Take HPMC and add 300ml of distilled water to dissolve, pass through 80 mesh sieve, remove insoluble matter, then add rizatriptan benzoate, glycerin, Tween-80, stir to dissolve. Add the finely ground titanium dioxide and aspartam into the slurry, stir, and leave overnight for vacuum degassing. Then stretch the membrane solution on a stainless steel belt, dry at 60°C, emboss with an embossing roller, cut it into a certain size, peel it off from the stainless steel belt, and seal the package.
Embodiment 2
[0072] Rizatriptan Benzoate 30g
[0073] HPMC 59.9g
[0074] PEG400 5g
[0075] Titanium dioxide 3g
[0076] Acesulfame K 2g
[0077] Sunset Yellow 60 0.1g
[0078] Take HPMC and add 300ml of distilled water to dissolve, pass through 80 mesh sieve to remove insoluble matter, then add rizatriptan benzoate, PEG 400, acesulfame potassium and sunset yellow 60, stir to dissolve. Add finely ground titanium dioxide into the slurry, stir, and leave overnight for vacuum degassing. Then stretch the membrane solution on a stainless steel belt, dry at 60°C, emboss with an embossing roller, cut it into a certain size, peel it off from the stainless steel belt, and seal the package.
Embodiment 3
[0080] Rizatriptan Benzoate 20g
[0081] PVA 46g
[0082] Glycerin 15g
[0083] Titanium dioxide 1g
[0085] Citric acid 2g
[0086] L-menthol 1g
[0087] Take PVA and add 100ml of distilled water to dissolve, pass through an 80-mesh sieve to remove insoluble matter, then add rizatriptan benzoate, glycerin, sodium saccharin and citric acid, and stir to dissolve. L-menthol was dissolved in ethanol and added to the film-forming slurry. Add finely ground titanium dioxide into the slurry, stir, and leave overnight for vacuum degassing. Then stretch the membrane solution on a stainless steel belt, dry at 60°C, emboss with an embossing roller, cut it into a certain size, peel it off from the stainless steel belt, and seal the package.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com