Didanosine pro-medicament and preparation method thereof
A technology of didanosine and prodrugs, which is applied in the field of medicine, can solve the problems of high polarity, only oral bioavailability, poor membrane permeability of the small intestine, etc., and achieve the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
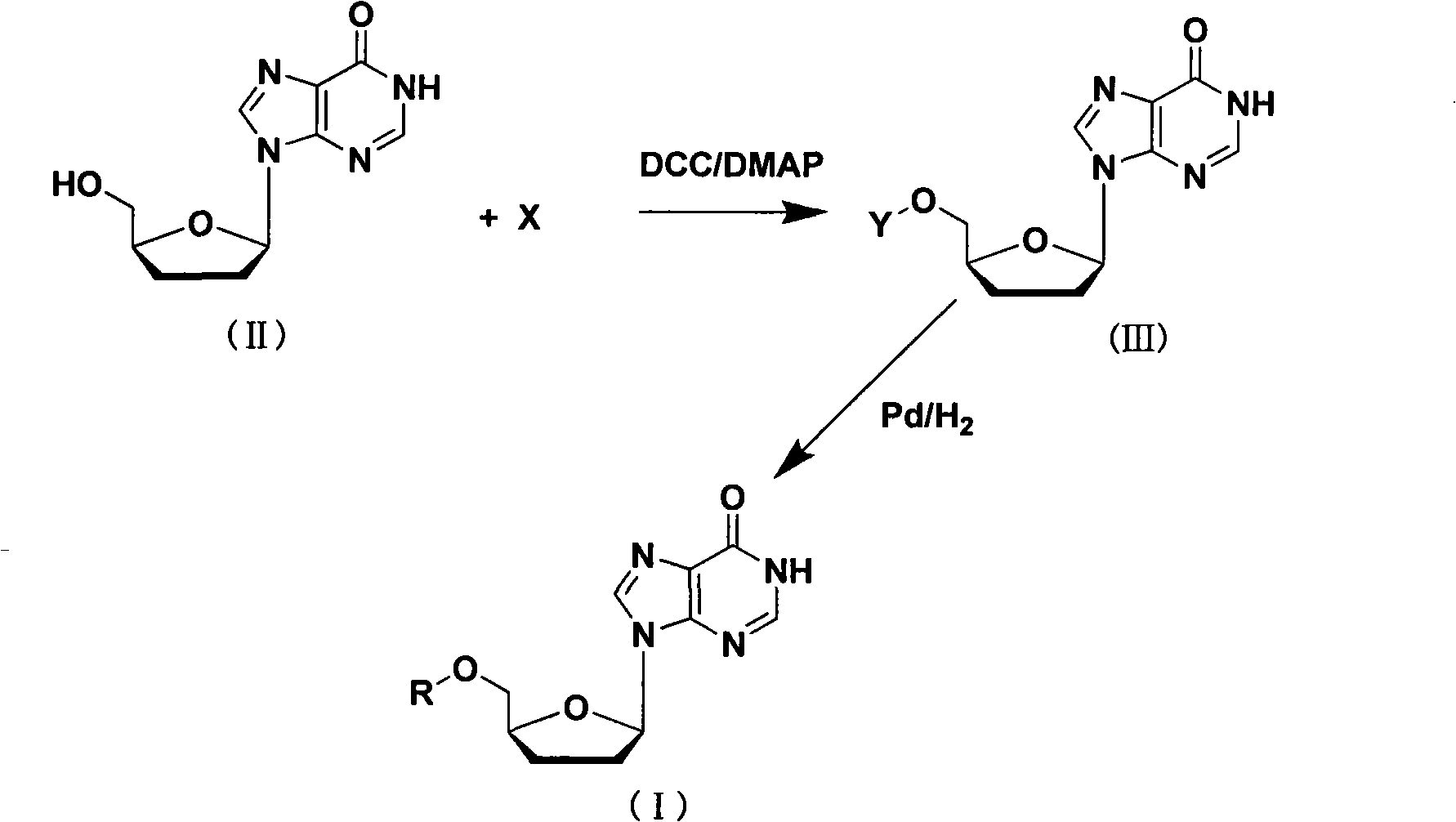
[0030] N-benzyloxycarbonyl-L-valine reacts with didanosine, adding dicyclohexylcarbodiimide and N,N-4-dimethylaminopyridine, the reaction solvent is anhydrous tetrahydrofuran, dichloromethane, cyclo Hexane or dimethylformamide, the reaction temperature is from 0°C to the boiling point of the solvent, preferably 20-60°C, and the reaction time is 8 hours; the reaction solution is cooled to room temperature, and the solvent is evaporated under reduced pressure. Extract with methyl chloride and wash with distilled water, saturated sodium bicarbonate and saturated brine successively, collect the organic layer, dry the column with sodium persulfate, after the filtrate is evaporated to dryness, recrystallize with ethyl acetate or dichloromethane, recrystallize the product, in acetic acid With ethyl acetate or dichloromethane as the solvent, add Pd / C as a catalyst, and perform catalytic hydrogenation under hydrogen conditions. The reaction time is 5 hours, suction filtration, and the filt...
Embodiment 2
[0032] N-benzyloxycarbonyl-L-isoleucine reacts with didanosine, adding dicyclohexylcarbodiimide and N,N-4-dimethylaminopyridine, the reaction solvent is anhydrous tetrahydrofuran, dichloromethane, Cyclohexane or dimethylformamide, the reaction temperature is 0°C to the boiling point of the solvent, preferably 20-60°C, and the reaction time is 8 hours; the reaction solution is cooled to room temperature, the solvent is evaporated under reduced pressure, and the residue is ethyl acetate or Extract with dichloromethane, and wash with distilled water, saturated sodium bicarbonate and saturated brine successively. Collect the organic layer, dry the column with sodium persulfate, and after the filtrate is evaporated to dryness, recrystallize with ethyl acetate or dichloromethane to recrystallize the product. With ethyl acetate or dichloromethane as the solvent, add Pd / C as a catalyst, and carry out catalytic hydrogenation under hydrogen conditions. The reaction time is 5 hours, suction ...
Embodiment 3
[0034] N-benzyloxycarbonyl-L-phenylalanine reacts with didanosine, adding dicyclohexylcarbodiimide and N,N-4-dimethylaminopyridine, the reaction solvent is anhydrous tetrahydrofuran, dichloromethane, Cyclohexane or dimethylformamide, the reaction temperature is 0°C to the boiling point of the solvent, preferably 20-60°C, and the reaction time is 8 hours; the reaction solution is cooled to room temperature, the solvent is evaporated under reduced pressure, and the residue is ethyl acetate or Extract with dichloromethane, and wash with distilled water, saturated sodium bicarbonate and saturated brine successively. Collect the organic layer, dry the column with sodium persulfate, and after the filtrate is evaporated to dryness, recrystallize with ethyl acetate or dichloromethane to recrystallize the product. With ethyl acetate or dichloromethane as the solvent, add Pd / C as the catalyst, and perform catalytic hydrogenation under hydrogen conditions. The reaction time is 5 hours, sucti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com