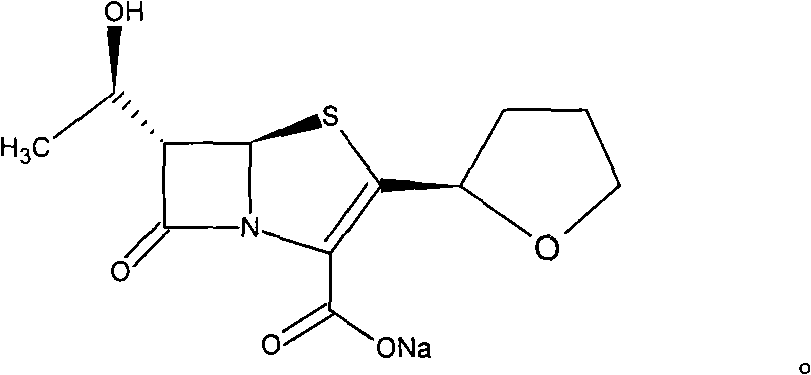
One-step synthesis method of Faropenem sodium
A technology of faropenem sodium, synthesis method, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry and other directions, can solve the problems of large catalyst dosage, unfavorable safety production and high cost, and achieves the The effect of color comparison, reduction of catalyst dosage, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Dissolve 49.0g of sodium bicarbonate in 1000g of water, add 33.0g of triethyl phosphite, 6.80g of palladium acetate, 47.0g of 5,5-dimethyl-1,3-cyclohexanedione and 500ml of dichloromethane, and stir 1h, add a solution of 188.7g of desiliconized matter and 1000ml of dichloromethane, raise the temperature to 35-37°C, keep stirring for about 3h, separate the water layer, add 1500ml of dichloromethane to wash once, separate the water layer, and add the active layer to the water layer Carbon 6g, decolorize 1h, filter, the filtrate is recovered to about 700ml of water, add dropwise 3000ml of acetone, precipitate and stir for 1h, filter to obtain a wet product, rinse with acetone 100ml*2, and obtain a white solid, that is, faropenem sodium 158.6g, yield 77.7 %. HPLC 99.6%. Heavy metal content <10ppm.
Embodiment 2
[0024] Dissolve 49.0 g of sodium bicarbonate in 1000 g of water, add 33.0 g of triethyl phosphite, 3.2 g of palladium acetate, 47.0 g of 5,5-dimethyl-1,3-cyclohexanedione and 500 ml of dichloromethane, and stir 1h, add a solution of 188.7g of desilicated matter and 1000ml of dichloromethane, heat up to 30-40°C, keep stirring for about 3h, separate the water layer, add 1500ml of dichloromethane to wash once, separate the water layers, and add activity to the water layer Carbon 6g, decolorize 1h, filter, the filtrate is recovered to about 700ml of water, add dropwise 3000ml of acetone, precipitate and stir for 1h, filter to obtain a wet product, rinse with acetone 100ml*2, and obtain a white solid, namely Faropenem Sodium 155.9g, yield 76.4 %. HPLC 99.5%. Heavy metal content <10ppm.
Embodiment 3
[0026] Dissolve 49.0g of sodium bicarbonate in 1000g of water, add 330g of triethyl phosphite, 3.2g of palladium acetate, 47.0g of 5,5-dimethyl-1,3-cyclohexanedione and 500ml of dichloromethane, and stir for 1h , add a solution of 188.7g of desiliconized matter and 1000ml of chloroform, raise the temperature to 30-40°C, keep stirring for about 3h, separate the water layer, add 1500ml of chloroform to wash once, separate the water layer, add 6g of activated carbon to the water layer, and decolorize for 1h , filtered, the filtrate was recovered to about 700ml of water, 3000ml of acetone was added dropwise, precipitated and stirred for 1h, the wet product was obtained by filtration, rinsed with acetone 100ml*2, and a white solid was obtained, namely 157.6g of faropenem sodium, with a yield of 77.3%. HPLC 99.6%. Heavy metal content <10ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com