Brominated PTP1B inhibitor as well as synthesis method and application thereof in preparation of medicine for curing type 2 diabetes
A kind of inhibitor, bromination technology, applied in the field of chemical total synthesis of brominated compound bis-methane, can solve the problems of few, poor chemical and biological stability, high electronegativity, etc., and achieve simple process operation and good industrialization Prospect of production, effect of reducing reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
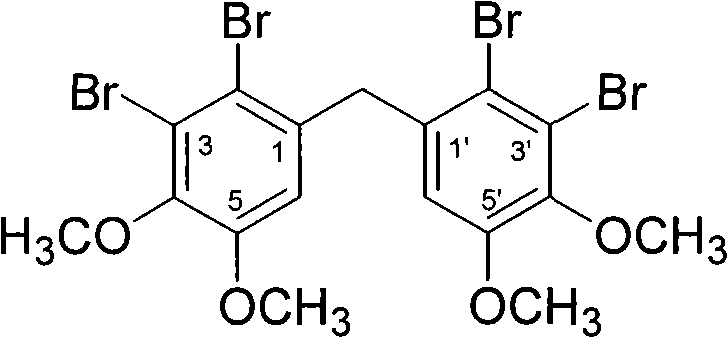
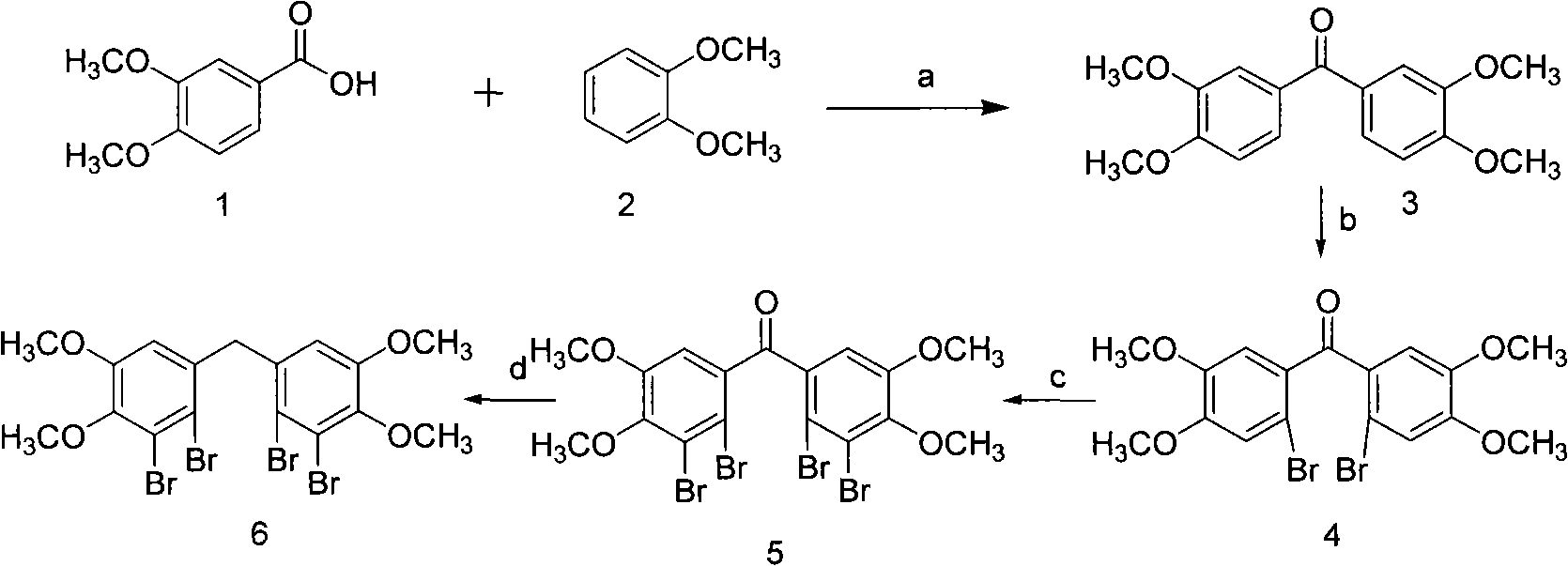
[0029] Example 1 The chemical synthesis and structural identification of "bis-(2,3-dibromo-4,5-dimethoxy-phenyl)-methane"
[0030] (1) Chemical synthesis and structure identification of Bis-(3,4-dimethoxy-phenyl)-methanone 3
[0031] Add 44.16g (240mmol) veratrol (compound 1), 33.84g (240mmol) veratrol (compound 2) and 200g polyphosphoric acid into a 1000ml three-necked reaction flask according to the molar ratio of 1:1, and stir at 80°C for reaction 1h; Cool to 60°C and add 500ml of ice water dropwise to the reactant within 30min, at this time, a large amount of water-insoluble pink solid precipitates out of the reactant; after removing water by filtration, dissolve the obtained solid in 200ml of dichloromethane , washed three times with an equal volume of 3% sodium hydroxide solution and distilled water successively; after drying the dichloromethane phase with anhydrous sodium sulfate, concentrate under reduced pressure to obtain a pink solid; wash the solid with petroleum e...
Embodiment 2
[0039] Example 2 Determination of protein tyrosine phospholipase 1B inhibitory activity
[0040] Prepare the test compound bis-(2,3-dibromo-4,5-dimethoxy-phenyl)-methane with DMSO into different concentrations of the test solution, take 2 μL of the test solution and add it to the standard The bioassay system (50mM Tris-HCl, PH6.5, 2mM pNPP, 2% DMSO, 30nM hGST-PTP1B), negative control: DMSO, positive control: sodium orthovanadate, the reaction temperature is 30°C, and the dynamic measurement wavelength is The light absorption at 405nm takes 3 minutes, and the inhibition rate of compound PTP1B enzyme activity is calculated according to the following formula. Inhibition rate=(experimental group A value-negative control group A value) / (control group A value-negative control group A)×100%, the results are shown in Table 1.
[0041] Table 1 protein tyrosine phospholipase 1B inhibition rate (%)
[0042] Table 1 Inhibitory Ratio (%) of PTP1B
[0043]
[0044] The test results sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com