Preparation method of recombination human thymosin beta 16
A thymosin and recombinant protein technology, applied in the field of bioengineering, can solve the problems of environmental pollution and high cost, and achieve the effects of broad application prospects, long duration of action, and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
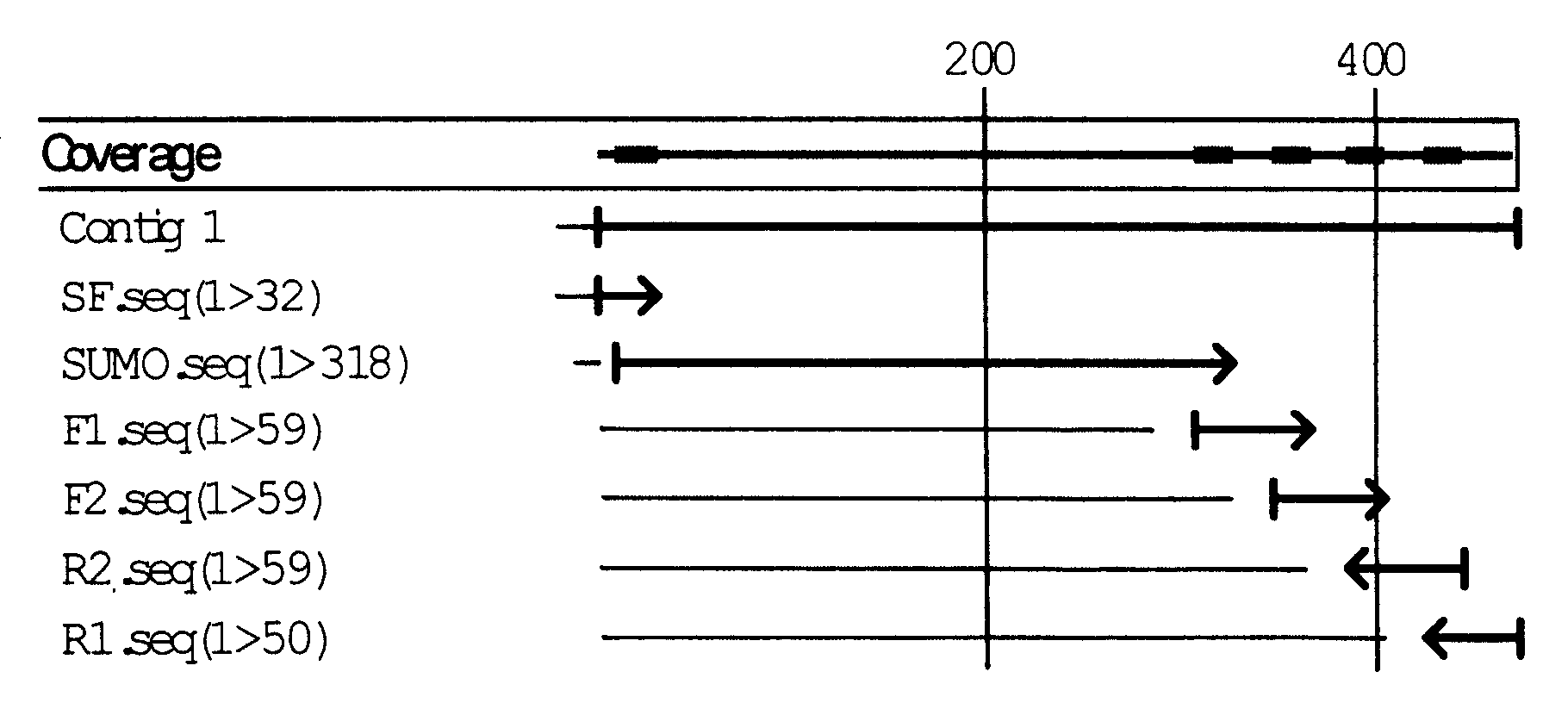
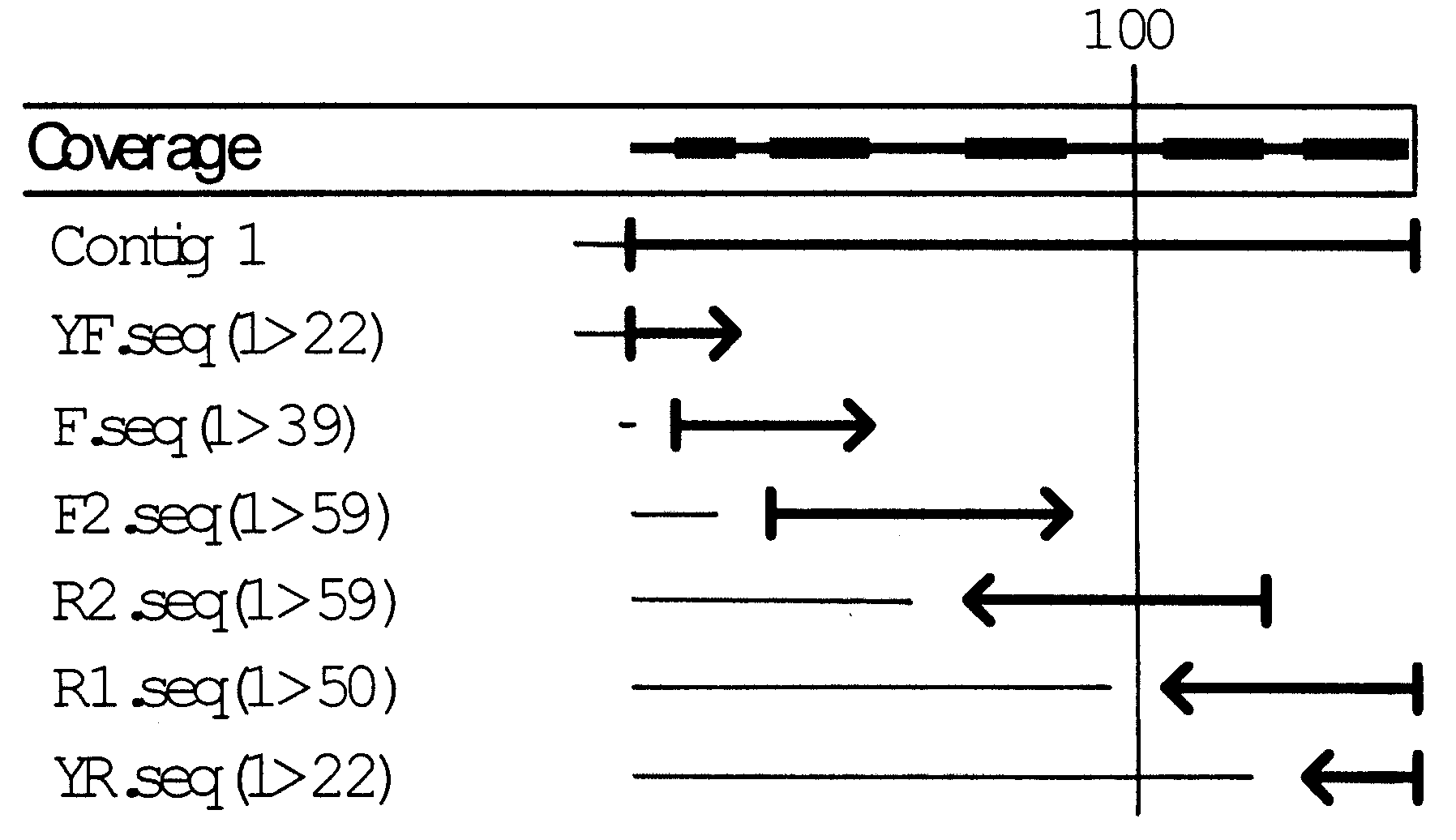
Image
Examples
Embodiment 1
[0052] Embodiment 1: pET / SUMO-Tβ16 fusion protein gene and its expression in Escherichia coli
[0053] 1. Reagents and materials
[0054] The vector plasmid pET3c was donated by the Engineering Research Center of the Ministry of Education for Bioreactor and Drug Development, the BL21 (DE3) strain was preserved by our laboratory, His, SUMO, and Tβ16 genes were synthesized by Shanghai Bioengineering Co., Ltd., and the DNA rapid purification / recovery kit was purchased from Beijing Dingguo Biotechnology Co., Ltd., e.z.N.A TM Plasmid Mini Kit I (100) plasmid extraction kit was purchased from OMEGA bio-tek company, Taq DNA polymerase, T 4 DNA ligase and restriction enzymes Nde I and BamH I were purchased from Bao Biological Engineering (Dalian) Co., Ltd.
[0055] 2. Method
[0056] 2.1 Design and synthesis of oligonucleotide primers:
[0057] The DNA sequences of SUMO and Tβ16 were designed with Escherichia coli preferred codons, the upstream primers contained Nde I restriction ...
Embodiment 2
[0076] Example 2: pGEX / GST-Tβ16 fusion protein gene and its expression in Escherichia coli
[0077] 1. Reagents and materials
[0078] The vector plasmid pGEX-6P-1 was donated by the Department of Agriculture of Jilin University, the BL21(DE3) strain was preserved by our laboratory, His, SUMO, and Tβ16 genes were synthesized by Shanghai Bioengineering Co., Ltd., and the DNA rapid purification / recovery kit was purchased from Beijing Dingguo Biotechnology Technology LLC, e.z.N.A TM Plasmid Mini Kit I (100) plasmid extraction kit was purchased from OMEGAbio-tek company, Taq DNA polymerase, T 4 DNA ligase and restriction enzymes EcoR I and Sal I were purchased from Bao Biological Engineering (Dalian) Co., Ltd.
[0079] 2. Method
[0080] 2.1 Design and synthesis of oligonucleotide primers:
[0081] The DNA sequences of SUMO and Tβ16 were designed with Escherichia coli preferred codons, the upstream primers contained EcoR I restriction sites, and the downstream primers contained ...
Embodiment 3
[0100] Example 3: pTW-Tβ16 fusion protein gene and its expression in Escherichia coli
[0101] 1. Reagents and materials
[0102] The vector plasmid pTWIN1, the expression strain E.coli ER2566, the endonuclease Sap I and chitin were purchased from NEB Company; the Tβ16 gene was synthesized by Shanghai Bioengineering Co., Ltd., and the DNA rapid purification / recovery kit was purchased from Beijing Dingguo Biotechnology Co., Ltd. Responsible Company, e.z.N.A TM Plasmid Mini Kit I (100) plasmid extraction kit was purchased from OMEGAbio-tek company, Taq DNA polymerase, T 4 DNA ligase was purchased from Bao Biological Engineering (Dalian) Co., Ltd.
[0103] 2. Method
[0104] 2.1 Design and synthesis of oligonucleotide primers:
[0105] The DNA sequence of Tβ16 was designed with E. coli preferred codons as follows:
[0106]
PF (on)
5′-AACATGAGTGATAAGC-3′
F
ATGAGTGATAAGCCAGATCTTAGTGAAGTTGAAAAAGAA
F2
TTAGTGAAGTTGAAAAAGAAGATCGCAGTAAGCTT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com