Specific binding protein and application thereof
A composition and monoclonal antibody technology, applied in the medical field, can solve problems such as low EGFRvIII specificity and unsatisfactory antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] The preparation of embodiment 1 antigen
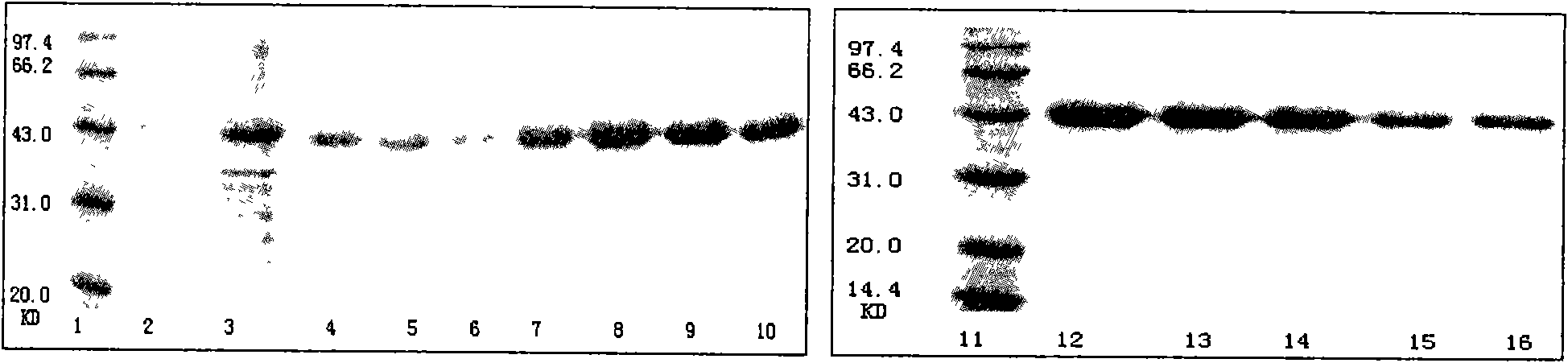
[0086] 1.1 Prokaryotic expression and purification of the extracellular domain of EGFRvIII protein
[0087] 1.1.1 Vector construction and identification
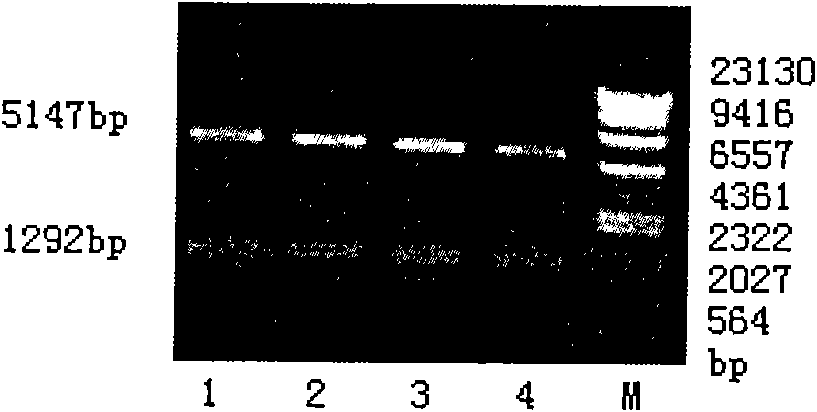
[0088] Using pLNRNL (encoding full-length EGFRvIII, purchased from Ludwig Institute, San Diego, CA) as a template, the amplification product of EGFRvIIIex with restriction sites BamHI and SalI at both ends was obtained by PCR method, and double-digested with BamHI and SalI, Get the target fragment. The commercially available vector pET28a (available from Novagen) was digested with BglII and SalI, the target fragment was recovered after agarose gel electrophoresis, ligated under the action of T4 ligase to form the vector pET28a-EGFRvIIIex, and then transformed into the commercially available large intestine Bacillus TOP10 (available from Invitrogen) was screened by Kana resistance, and positive clones containing insert fragments were identified by BglII and SalI digestion.
...
Embodiment 2
[0110] Example 2. Antigen immunization and hybridoma screening
[0111] 2.1 Immunity
[0112] (1) Recombinant protein immunization:
[0113] The EGFRvIII extracellular region recombinant protein was fully emulsified and mixed with an equal amount of complete Freund's adjuvant (Sigma) to subcutaneously immunize 6-week-old BALB / c mice, 100 μg per mouse. Four weeks later, the recombinant antigen was emulsified and mixed with incomplete Freund's adjuvant, and the mice were immunized by intraperitoneal injection, 50 μg per mouse, and the intraperitoneal booster immunization was continued at intervals of 2 weeks thereafter. One week after the fourth booster immunization, the recombinant antigen was coated, and the antiserum titer of the mouse was detected by ELISA method > 10 5 .
[0114] (3) Intrasplenic injection to boost immunity:
[0115] Three weeks after the last boost, 20 μg of recombinant antigen was immunized intrasplenicly.
[0116] 2.2 Establishment of hybridoma cell...
Embodiment 3
[0128] Embodiment 3 Detection of binding ability of monoclonal antibody
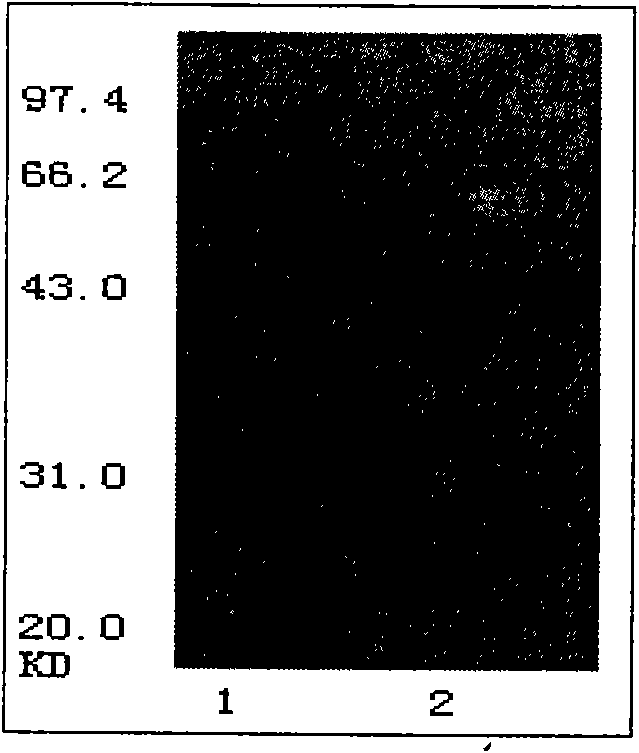
[0129] 3.1 FACS analysis of receptor binding specificity of 12H23
[0130] Vector build:
[0131] Cells: U87 cells (glioma cell line, normal expression of EGFR, can be purchased from ATCC cell bank), U87-EGFRvIII cells (U87 cell line transfected with pLERNL vector) and A431 cells (human epidermal squamous cell carcinoma, Overexpression of EGFR, available from ATCC cell bank)
[0132] Antibody: the antibody 12H23 prepared in Example 2, and the commercially available C225 monoclonal antibody (as a control). The antibody concentration was 2mg / ml, diluted 1:100.
[0133] 1) The cells in the logarithmic growth phase were inoculated into a 6-well plate at a density of about 90%, and cultured overnight in a 37°C incubator.
[0134] 2) The next day, digest the cells with 10 mM EDTA, centrifuge at 5000 rpm for 3 min, and collect the cells in a 2 ml Eppendorf tube.
[0135] 3) Cells were resuspended in 0.5-1m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com