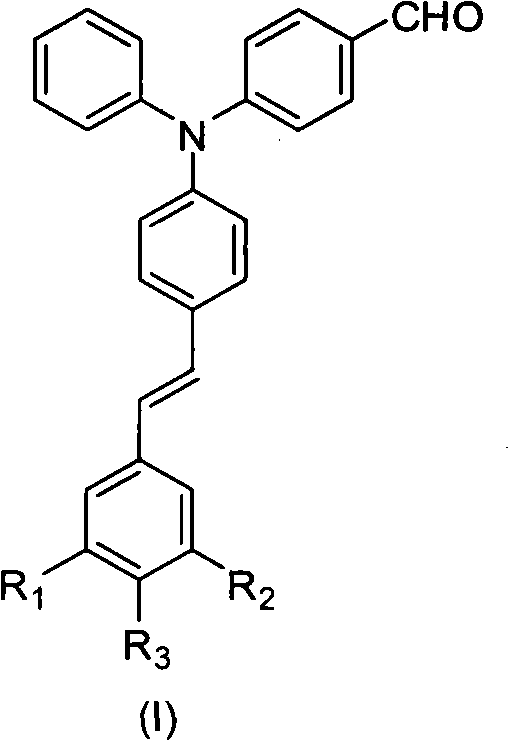
Aldehyde group-containing visible light photosensitizer taking triphenylamine as core and synthesis and application thereof
A triphenylamine and visible light technology, applied in the preparation of organic compounds, organic chemistry, carboxylic acid nitrile preparation, etc., can solve the problems of poor solubility of dyes and the need to improve the initiation efficiency, and achieve convenient raw material sources and appropriate yield , The effect of simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
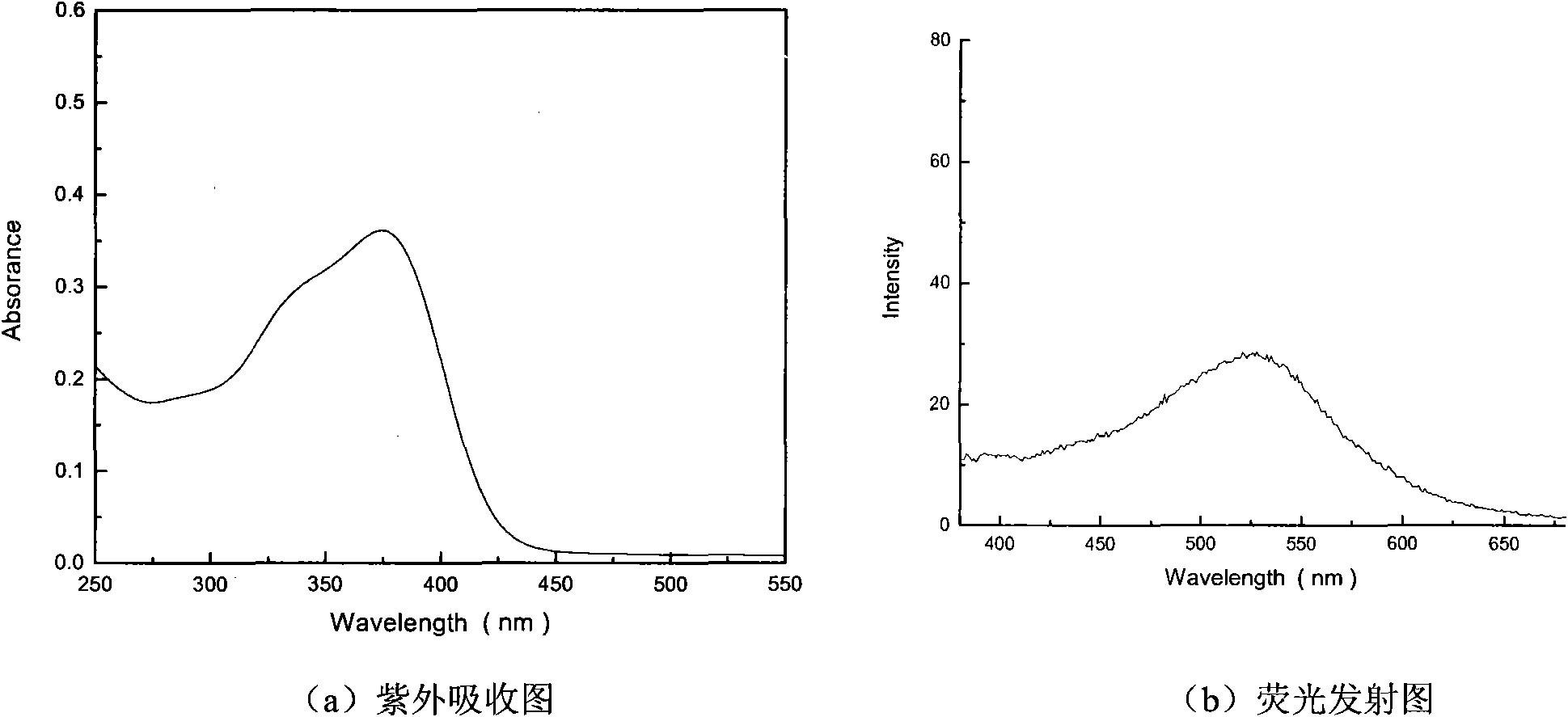
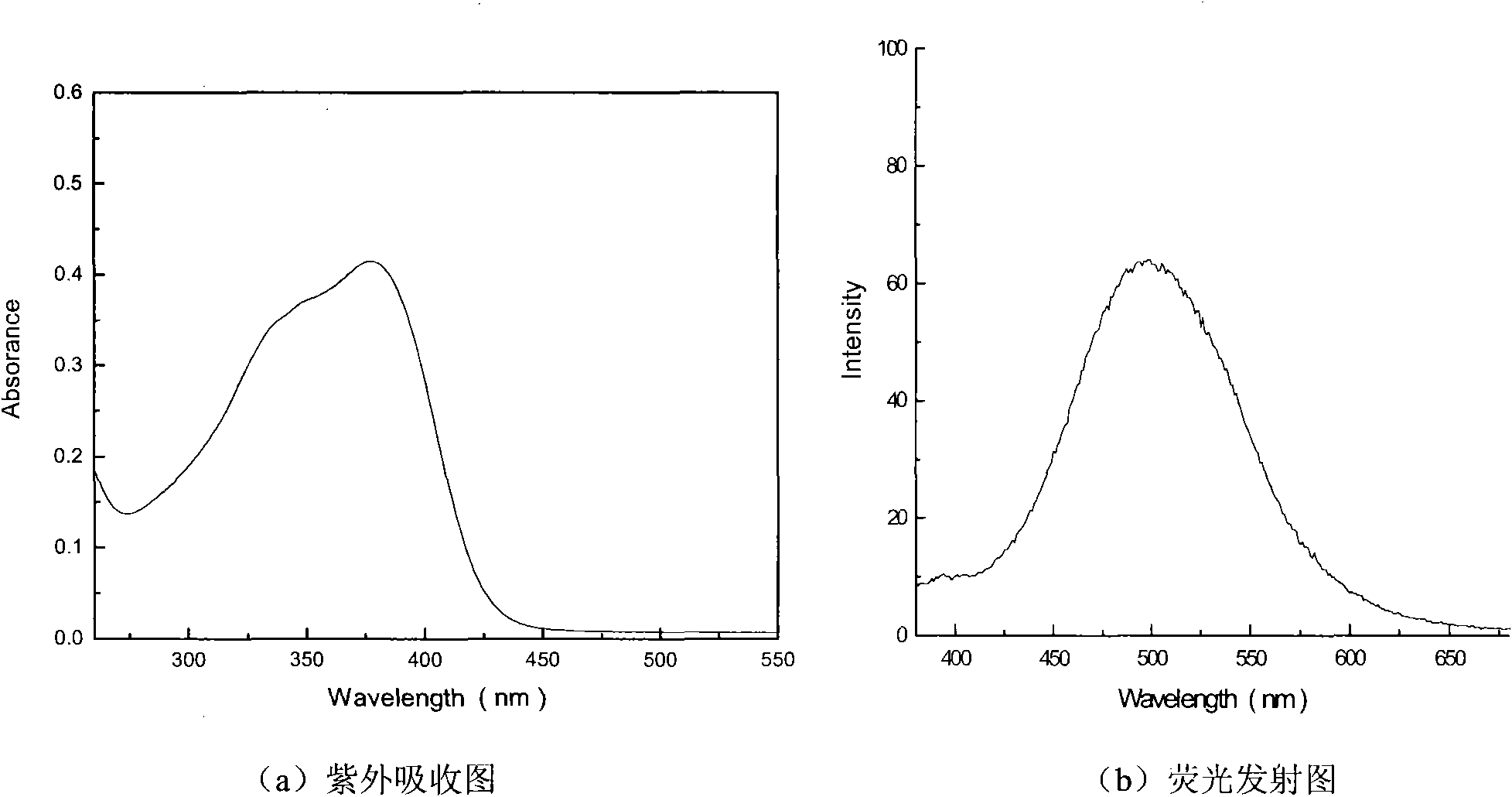
Image
Examples
Embodiment 1
[0031] Synthesis of N-phenyl-N-(distyryl)yl-4-benzaldehyde
[0032] The synthesis proceeds in three steps:
[0033] (1) Bis-(4-formylphenyl)-aniline
[0034] Add 25ml of N,N-dimethylformamide into a 250ml three-necked flask under an ice-water bath, and slowly add 20ml of phosphorus oxychloride dropwise under the protection of argon. After the dropwise addition, 3 g of triphenylamine was added, and the reaction was refluxed at 95° C. for 4 h. After the reaction is completed, cool to room temperature, add 200ml of distilled ice water, and adjust the pH to 7-8. Pour into 200ml of dichloromethane for extraction, then extract with distilled water 3 times, and dry over anhydrous sodium sulfate. Dichloromethane was evaporated to dryness, and the product was separated by a chromatographic column, concentrated, and recrystallized (ethyl acetate:cyclohexane=1:1) to obtain a yellow solid with a yield of 43.81%, which was set aside;
[0035] (2) Synthesis of diethyl phenylphosphonate ...
Embodiment 2
[0040] Synthesis of N-phenyl-N-(4-methylstilbene)yl-4-benzaldehyde
[0041] The synthesis proceeds in three steps:
[0042] (1) Bis-(4-formylphenyl)-aniline
[0043] Synthesis is carried out by the first step of embodiment 1;
[0044] (2) Synthesis of 4-methylphenylphosphonic acid diethyl ester
[0045] Mix 0.57g of 4-methylbenzyl bromide and 3.02g of triethyl phosphite in a three-necked ground flask with a molar ratio of 1:10, add a condensing device, and react at 130°C-160°C for 6 hours. Excessive triethyl phosphite was distilled under pressure to obtain diethyl 4-methylphenylphosphonate with a yield of 80%;
[0046] (3) Synthesis of N-phenyl-N-(4-methylstilbene)-4-benzaldehyde
[0047] Add 1.50g of bis-(4-formylphenyl)-aniline and 0.62g of 4-methylphenylphosphonic acid diethyl ester (molar ratio 3:1) synthesized in step (1) into the three-necked flask, Dissolve in tetrahydrofuran solvent with a volume ratio of 100-500 times, add 0.44g sodium methoxide, react under argo...
Embodiment 3
[0049] Synthesis of N-phenyl-N-(3,4,5-trimethoxystilbene)yl-4-benzaldehyde
[0050] The synthesis proceeds in three steps:
[0051] (1) Synthesis of two-(4-formylphenyl)-aniline
[0052] Synthesis is carried out by the first step of embodiment 1;
[0053] (2) Synthesis of 3,4,5-trimethoxyphenyl phosphonic acid diethyl ester
[0054] Mix 1.02g of 3,4,5-trimethoxybenzyl bromide and 4.47g of triethyl phosphite into a three-neck flask at a molar ratio of 1:10, add a condensing device, and react 6 at 130°C-160°C Hours, the excessive triethyl phosphite was distilled under reduced pressure to obtain 3,4,5-trimethoxyphenyl phosphonic acid diethyl ester with a yield of 70%, which was set aside;
[0055] (3) Synthesis of N-phenyl-N-(3,4,5-trimethoxystilbene)-4-benzaldehyde
[0056] Add 0.60g of 3,4,5-trimethoxyphenyl phosphonic acid diethyl ester and 0.23g of basic catalyst sodium methoxide (molar ratio 1:2.5) into the three-necked flask, stir under ice-water bath for half an hour, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| full width at half maximum | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com