Method for preparing nickel hydrazine azide
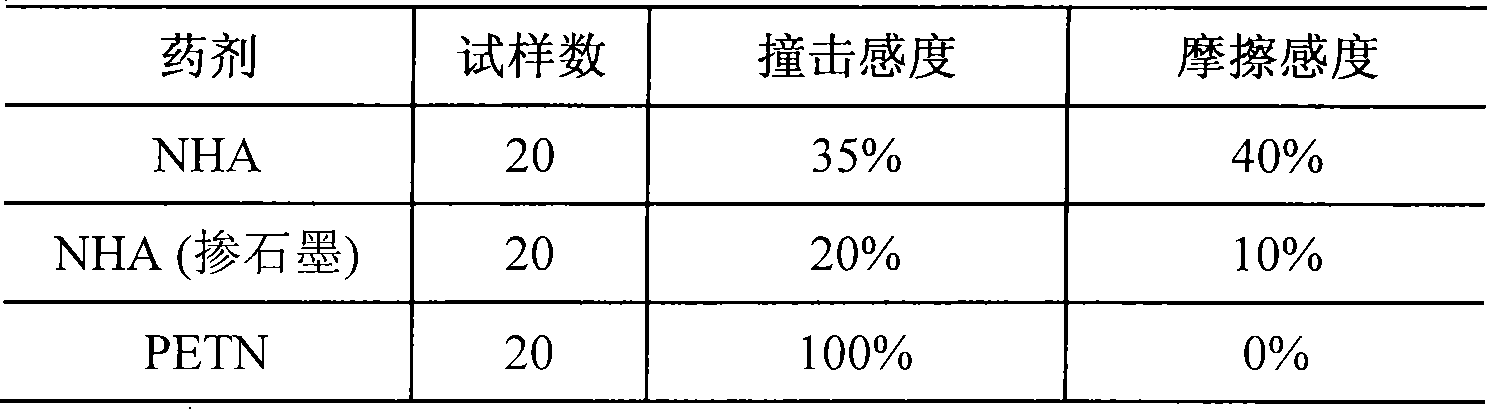
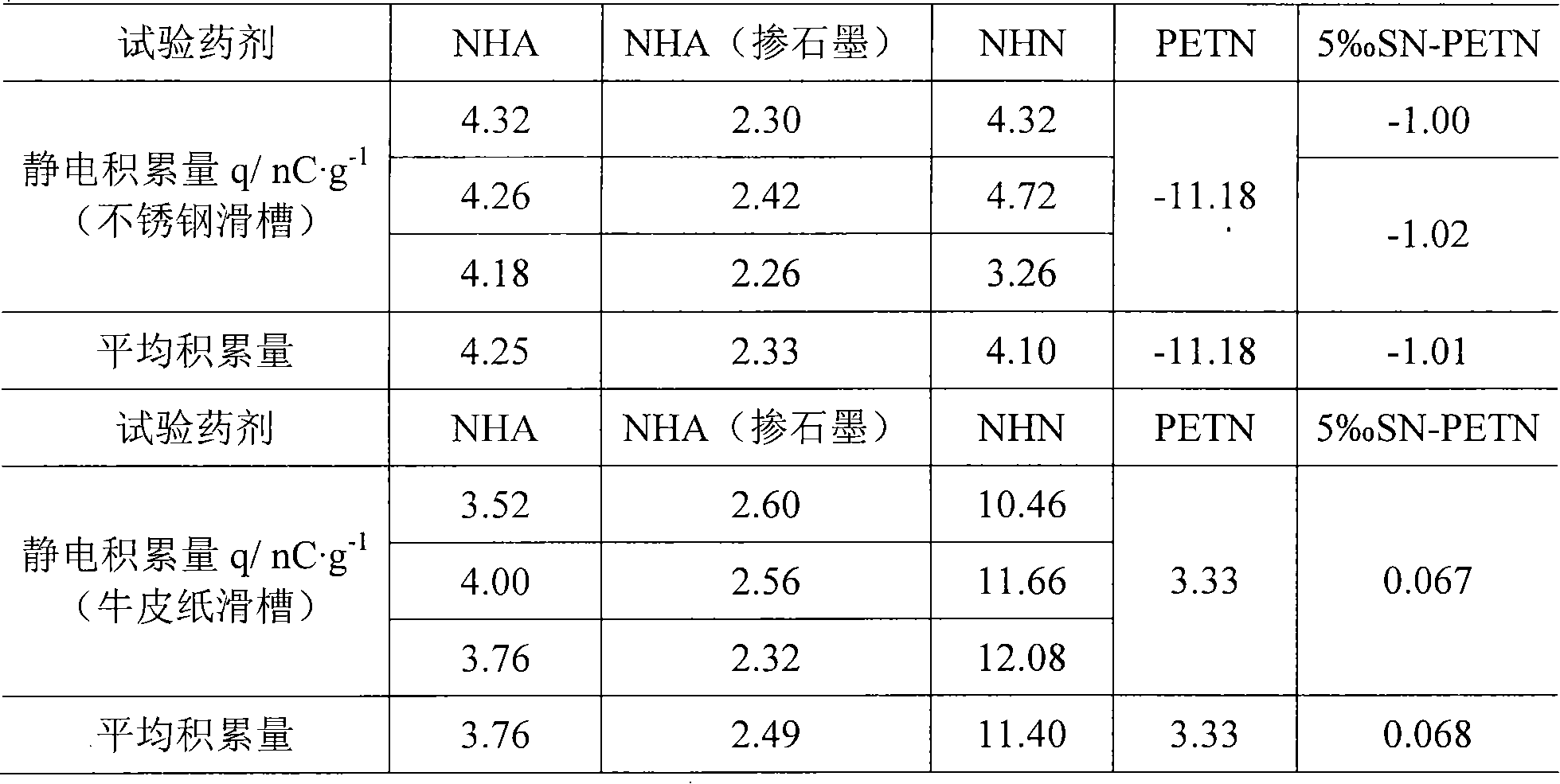
A technology of nickel salt and sodium nitride, which is applied in the direction of azide/azide/halogen azide, etc., can solve the problems affecting the feasibility and rationality of the process, environmental and personnel pollution, inconsistent drug quality, etc., and achieve Effects of reduced mechanical sensitivity, increased detonation ability, and stable pH
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
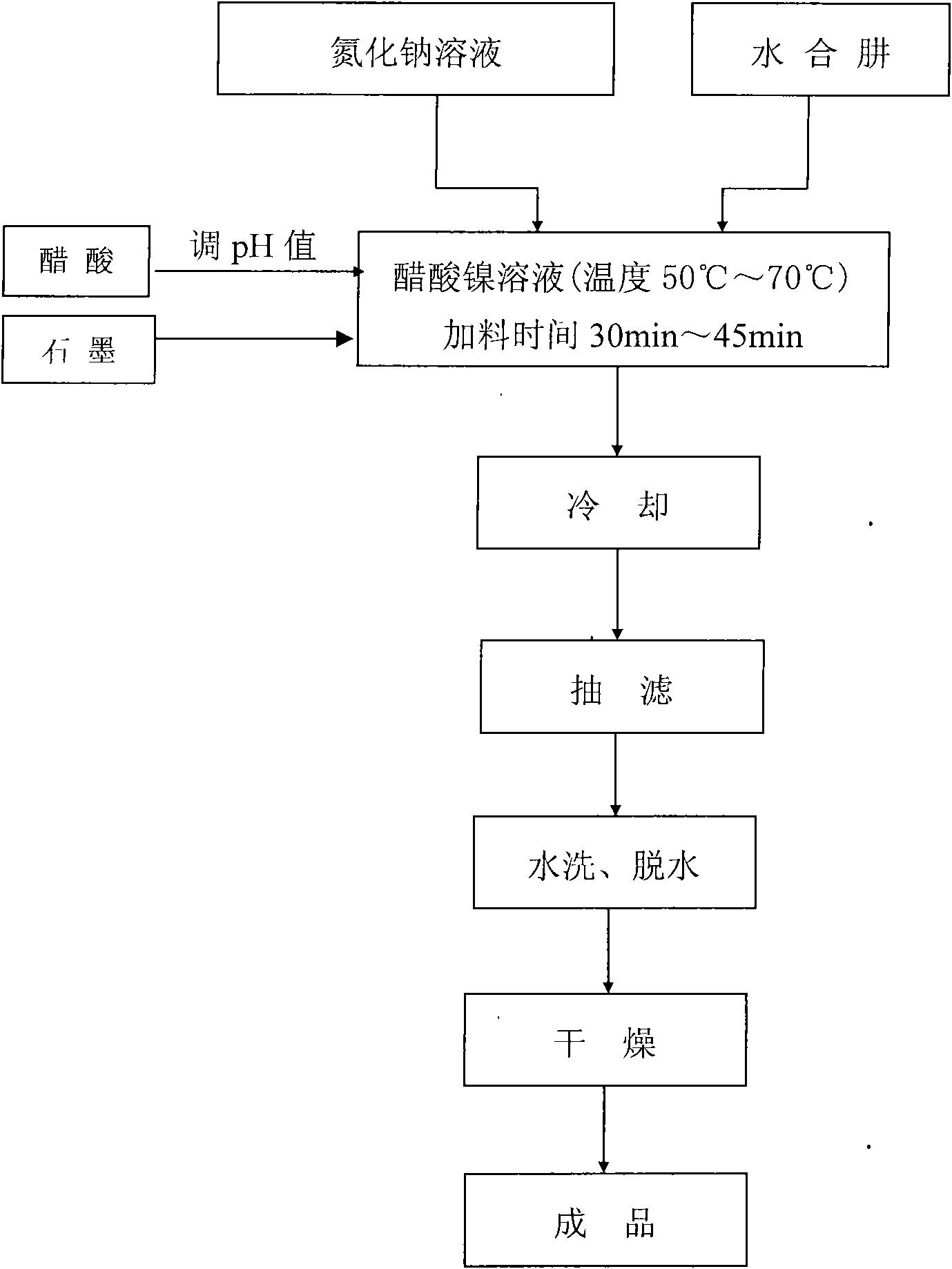
[0013] Embodiment 1: in conjunction with Fig. 1, raw material specification and requirement: hydrazine hydrate (N 2 h 4 ·H 2 O)AR, nickel acetate (NiAc 2 4H 2 O)CP, sodium nitride (NaN 3 ) CP, graphite 1000 mesh, acetic acid (HAc) CP, ethanol (C 2 h 5 OH)CP, main instruments and equipment: electric mixer; voltage regulating transformer; super constant temperature water bath; tall beaker (combiner) 400mL, metering pump, Buchner funnel Φ65mm; filter bottle 1000mL, water bath oven, water circulation vacuum pump.
[0014] According to the raw material feeding ratio (mass ratio): Ni(Ac) 2 4H 2 O:NaN 3 :N 2 h 4 ·H 2 O=1.76:1:0.80, weigh 12.3g of nickel acetate, dissolve it with 160mL of pure water, filter it and transfer it to a combiner, then add 4.0mL of acetic acid and 100mg of graphite; then weigh 7.0g of sodium nitride and measure Dissolve and dilute 6.0mL of hydrazine hydrate with 30mL of pure water, put them into small beakers after filtering, and set aside. Inst...
Embodiment 2
[0023] Embodiment 2: by raw material charging ratio (mass ratio): Ni(Ac) 2 4H 2 O:NaN 3 :N 2 h 4 ·H 2 O=1.76:1:0.80, weigh 14g of nickel acetate, dissolve it with 120mL of pure water, filter it and transfer it to the compounder, then add 3.5mL of acetic acid and 100mg of graphite; then weigh 7.0g of sodium nitride and measure 6.0 mL of hydrazine hydrate was dissolved and diluted with 90mL and 60mL of pure water respectively, filtered and put into small beakers respectively for later use. Install the reaction device and calibrate the metering pump. Heat in a constant temperature water bath to raise the temperature of the bottom liquid to 70°C, and at the same time start the stirrer and adjust the voltage to an appropriate stirring state. Slowly, uniformly and accurately add the sodium nitride and hydrazine hydrate solution dropwise through the metering pump, controlling the feeding time of sodium nitride to be 30min±5min, and the feeding time of hydrazine hydrate to be 45...
Embodiment 3
[0024] Embodiment 3: according to raw material charging ratio (mass ratio): Ni(Ac) 2 4H 2 O:NaN 3 :N 2 h 4 ·H 2 O=1.76:1:0.80, weigh 12.3g of nickel acetate, dissolve it with 120mL of pure water, filter it and transfer it to a combiner, then add 3.5mL of acetic acid and 100mg of graphite; then weigh 7.0g of sodium nitride and measure Dissolve and dilute 5.6mL of hydrazine hydrate with 30mL and 30mL of pure water respectively, put them into small beakers after filtering, and set aside. Install the reaction device and calibrate the metering pump. Heat in a constant temperature water bath to raise the temperature of the bottom solution to 65°C, and at the same time start the stirrer and adjust the voltage to an appropriate stirring state. Slowly, uniformly and accurately add the sodium nitride and hydrazine hydrate solution dropwise through the metering pump, controlling the feeding time of sodium nitride to be 30min±2min, and the feeding time of hydrazine hydrate to be 45m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com