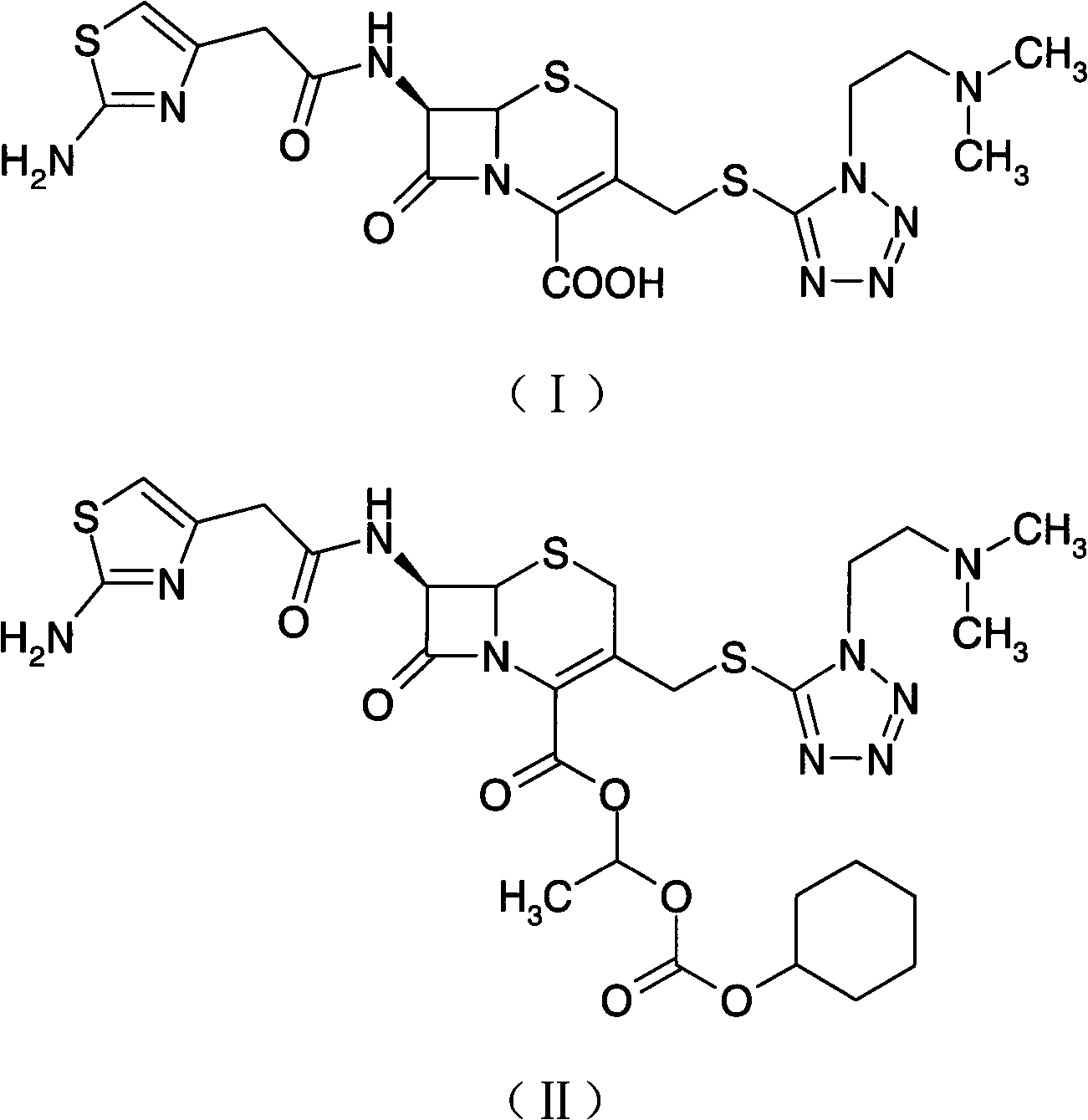
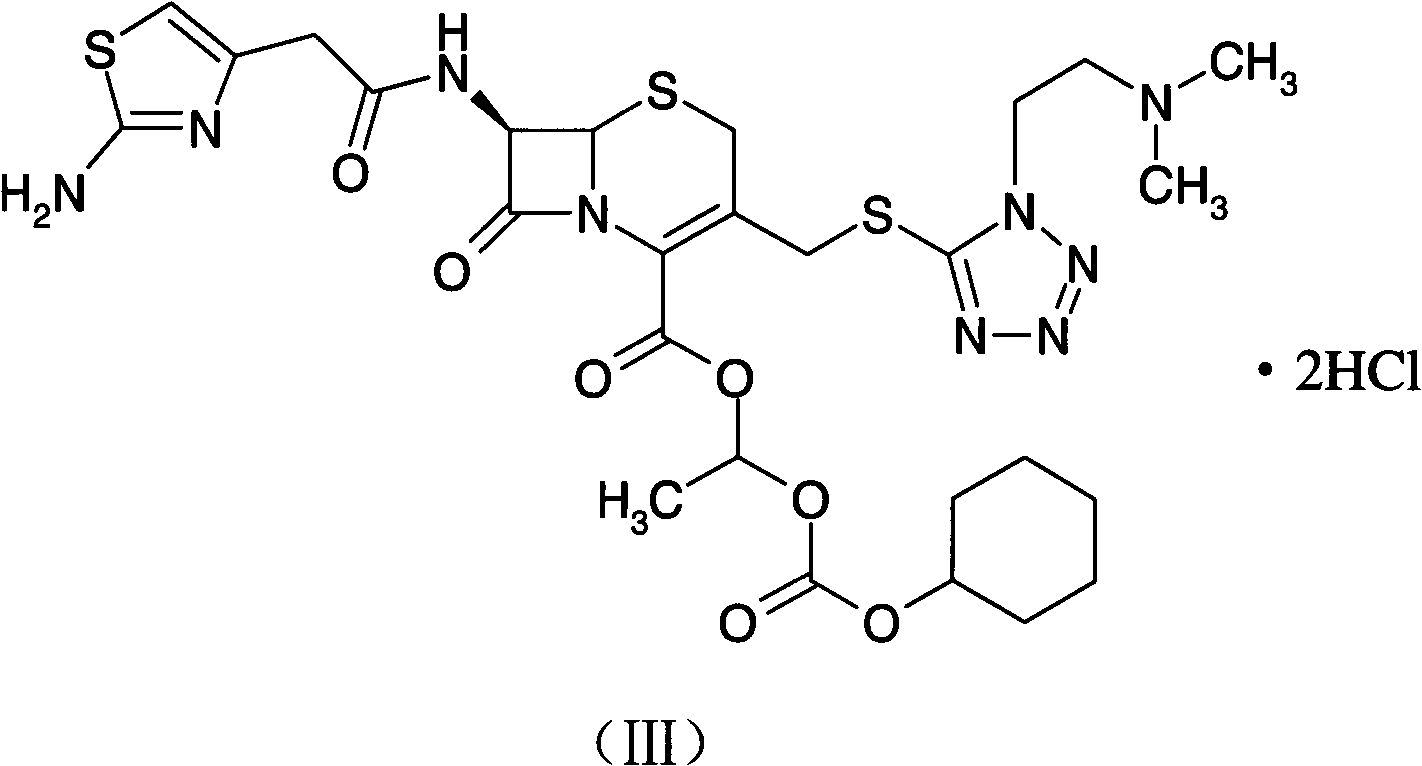
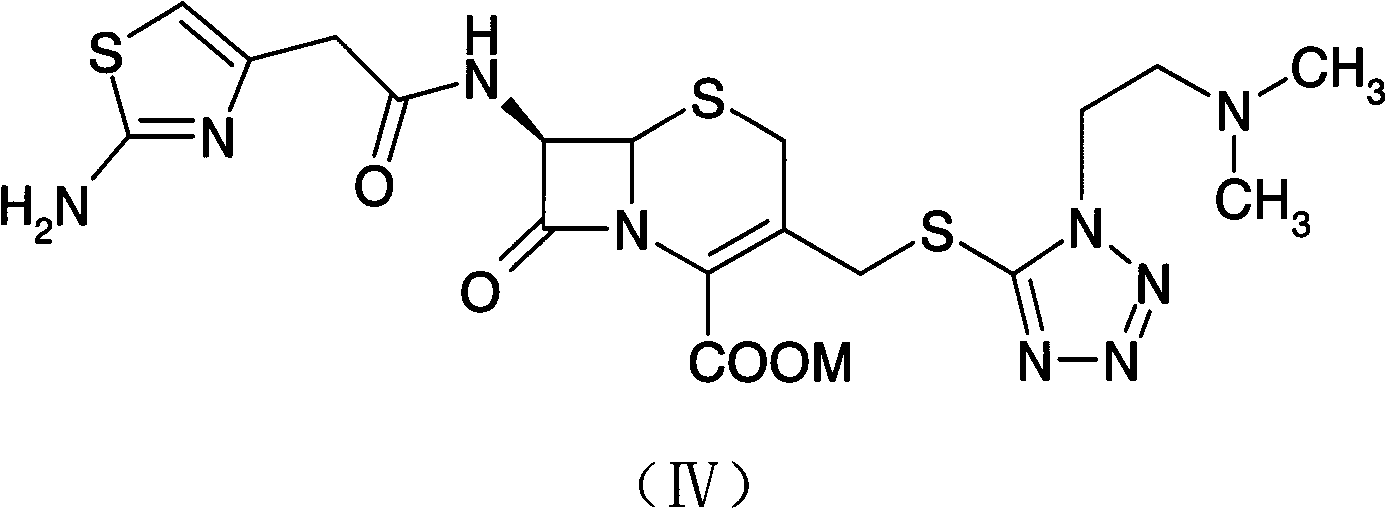
Preparation method of cefotiam hexetil hydrochloride
A technology of cefotiam and cefotiam, applied in the field of drug antibiotics, can solve the problems of unsuitability for large-scale industrial production, complex post-processing operations of products, high by-products, etc., and achieve the effect of low production cost, short time and long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1 cefotiam hydrochloride
[0045] The preparation method of the present embodiment cefotiam axetil hydrochloride, its concrete steps are as follows:
[0046] (1) Add 500ml of methanol into a 2000ml reaction bottle, cool down to -2°C, add 50g (0.084mol) of cefotiam hydrochloride, and dissolve to obtain a methanol solution of cefotiam hydrochloride; at the same time, dissolve potassium acetate into the methanol solution 5% methanol solution of potassium acetate was prepared in the method; 250ml (0.105mol) of 5% methanol solution of potassium acetate was taken, and slowly added dropwise to the methanol solution of cefotiam hydrochloride, the drop was completed within 1 hour, stirred for 30min, and 500ml of Propanol, stirred for 1 hour, crystallized at -5°C for 1 hour, filtered, and the filter cake was dried in a vacuum oven at 40°C until constant weight to obtain 39.4 g of cefotiam potassium salt;
[0047] (2) Add DMF (200ml) to a 500ml react...
Embodiment 2
[0052] The preparation of embodiment 2 cefotiam hydrochloride
[0053] The preparation method of the present embodiment cefotiam axetil hydrochloride, its concrete steps are as follows:
[0054] (1) Add 500ml of methanol into a 2000ml reaction flask, cool down to -2°C, add 50g (0.084mol) of cefotiam hydrochloride, and dissolve to obtain a methanol solution of cefotiam hydrochloride; at the same time, dissolve sodium acetate into the methanol solution Prepare 5% sodium acetate methanol solution; take 200ml of 5% sodium acetate methanol solution, slowly add it dropwise to cefotiam hydrochloride methanol solution, drop it within 1 hour, stir for 30min, add 500ml isopropanol, stir 1h, placed at -5°C for crystallization for 1 hour, filtered, and dried the filter cake in a vacuum oven at 40°C to constant weight to obtain 41.6g of cefotiam potassium salt;
[0055] (2) Add 200ml of DMF to a 500ml reaction bottle, cool down to 3°C, add 20g (0.035mol) of cefotiam potassium, stir until ...
Embodiment 3
[0060] The optimization of embodiment 3 extraction conditions
[0061] A preparation method of cefotamate hydrochloride, the method may further comprise the steps:
[0062] (1) cefotiam and acetate react to prepare cefotiam salt;
[0063] (2) the cefotiam salt prepared by step (1) is subjected to an esterification reaction with 1-iodoethyl cyclohexyl carbonate in the presence of potassium carbonate;
[0064] (3) After the esterification reaction of step (2) ends, add an organic solvent to the reaction solution, keep the organic solvent layer after extraction; add hydrochloric acid to the organic solvent layer, and collect the hydrochloric acid layer after extraction;
[0065] (4) The pH of the hydrochloric acid layer obtained in step (3) is adjusted to weak acidity, then extracted with an organic solvent, and the organic layer extract is collected;
[0066] (5) Crystallize and filter the organic layer extract obtained in step (4) to obtain cefotiam axetil hydrochloride.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com