Method for preparing tin-nickel alloy of cathode materials of lithium ion battery by electrolyzing melted salt
A technology for lithium-ion batteries and negative electrode materials, applied in battery electrodes, electrode manufacturing, circuits, etc., can solve problems such as performance degradation, achieve the effects of reducing energy consumption, accelerating electrolytic reaction speed, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
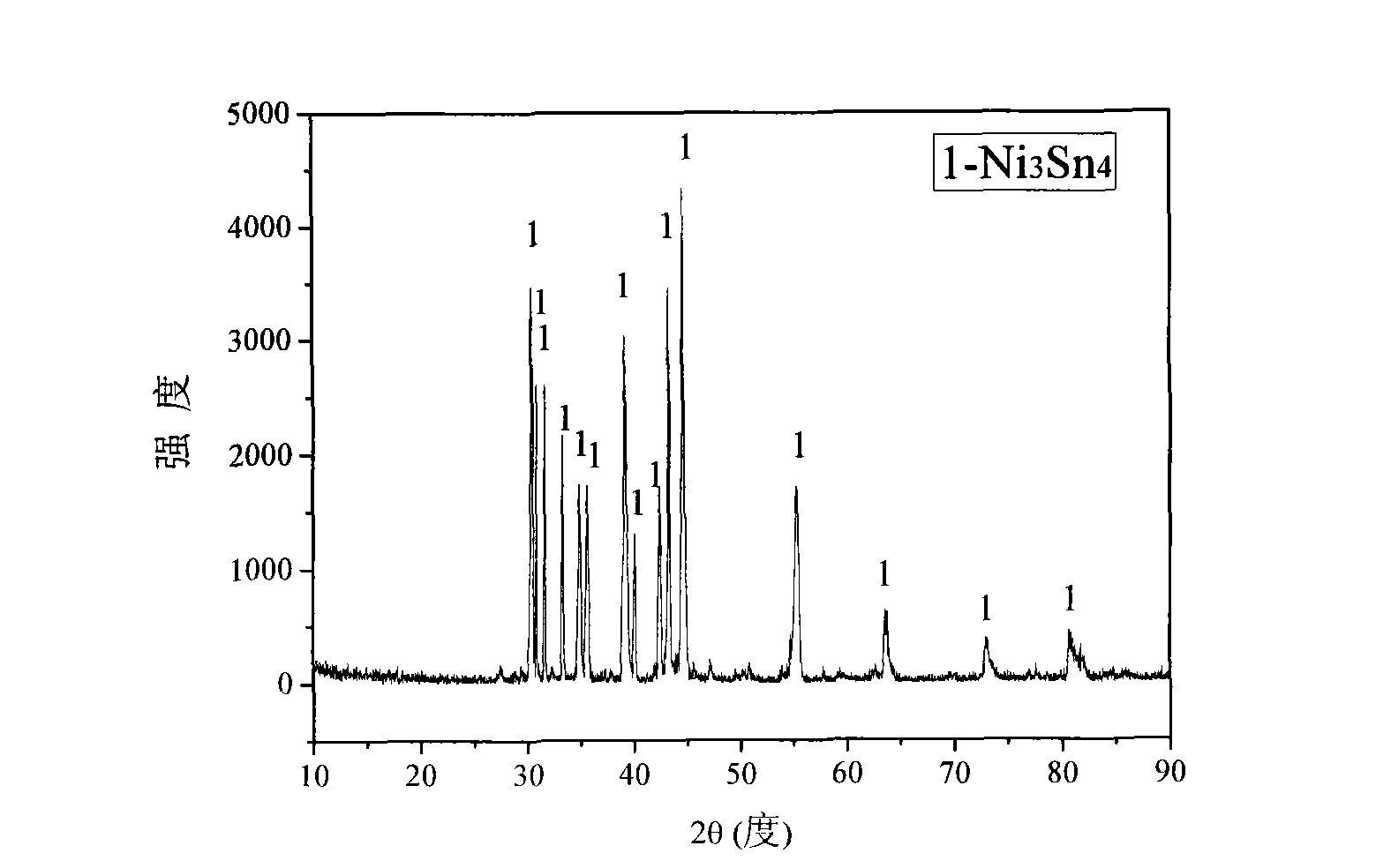
[0024] Will analyze pure NiO and SnO 2 The powder is mixed according to the molar ratio of 3:4, adding 3-10wt% graphite powder, wet milling in a ball mill for 3 hours, taking out and drying, weighing 2.5g of the sample, and then pressing the powder into a diameter of 10mm under 30MPa, A cylinder with a height of 3.5mm, and sintered at 850-1250°C for 2-4 hours to obtain a sintered oxide sheet; hang the sintered oxide sheet on an iron-chromium-aluminum wire, and wrap the periphery with an iron-chromium-aluminum wire, as Cathode; High-purity graphite rod is used as anode. Insert it into the CaCl 2 In the alumina crucible mixed with NaCl molten salt, under the protection of Ar gas, the electrolysis is carried out at a controlled voltage of 2.3-3.2V. The electrolysis temperature is 600-750°C. After 6-10 hours of electrolysis, the electrolysis is ended; cooling in an inert atmosphere After washing with distilled water and drying, the product is pure phase Ni 3 sn 4 alloy.
[00...
Embodiment 2
[0027] Will analyze pure NiO and SnO 2 The powder is mixed in a molar ratio of 1:1, and 5-15wt% of CaCO is added 3 , wet milled in a ball mill for 3 hours, took out and dried, then weighed 2.5g of the sample, pressed the powder into a cylinder with a diameter of 10mm and a height of 3.5mm at 30MPa, and sintered it at 850-1250°C for 3-8 Hours, the oxide sintered sheet is obtained; the oxide sintered sheet is suspended on the iron-chromium-aluminum wire, and the periphery is wrapped with the iron-chromium-aluminum wire as the cathode; a high-purity graphite rod is used as the anode. Insert it with CaCl 2 In the alumina crucible mixed with NaCl molten salt, under the protection of Ar gas, the electrolysis is carried out at a controlled voltage of 2.0-3.2V, and the electrolysis temperature is 650-780°C. After 5-8 hours of electrolysis, the electrolysis is ended; it is cooled in an inert atmosphere After washing with distilled water and drying, the product is Ni 3 sn 4 / Ni 3 s...
Embodiment 3
[0030] Will analyze pure NiO and SnO 2 The powder is mixed according to the molar ratio of 2:1, 3-15wt% of starch is added, wet-milled in a ball mill for 3 hours, taken out and dried, then weighed 2.5g of the sample, and pressed the powder into a diameter of 10mm under 30MPa, with a height of 3.5mm cylinder, and sinter it at 850-1250°C for 2-4 hours to obtain oxide sintered sheet; hang the oxide sintered sheet on molybdenum wire, and wrap the periphery with molybdenum wire as the cathode; adopt high-purity Graphite rods serve as anodes. Insert it into the CaCl 2 In the alumina crucible mixed with NaCl molten salt, under the protection of Ar gas, the electrolysis is carried out at a controlled voltage of 2.0-3.2V, and the electrolysis temperature is 600-780°C. After 8-16 hours of electrolysis, the electrolysis is ended; cooling in an inert atmosphere After washing with distilled water, and drying, the product is Ni / Ni 3 sn 4 / Ni 3 sn 2 alloy compound.
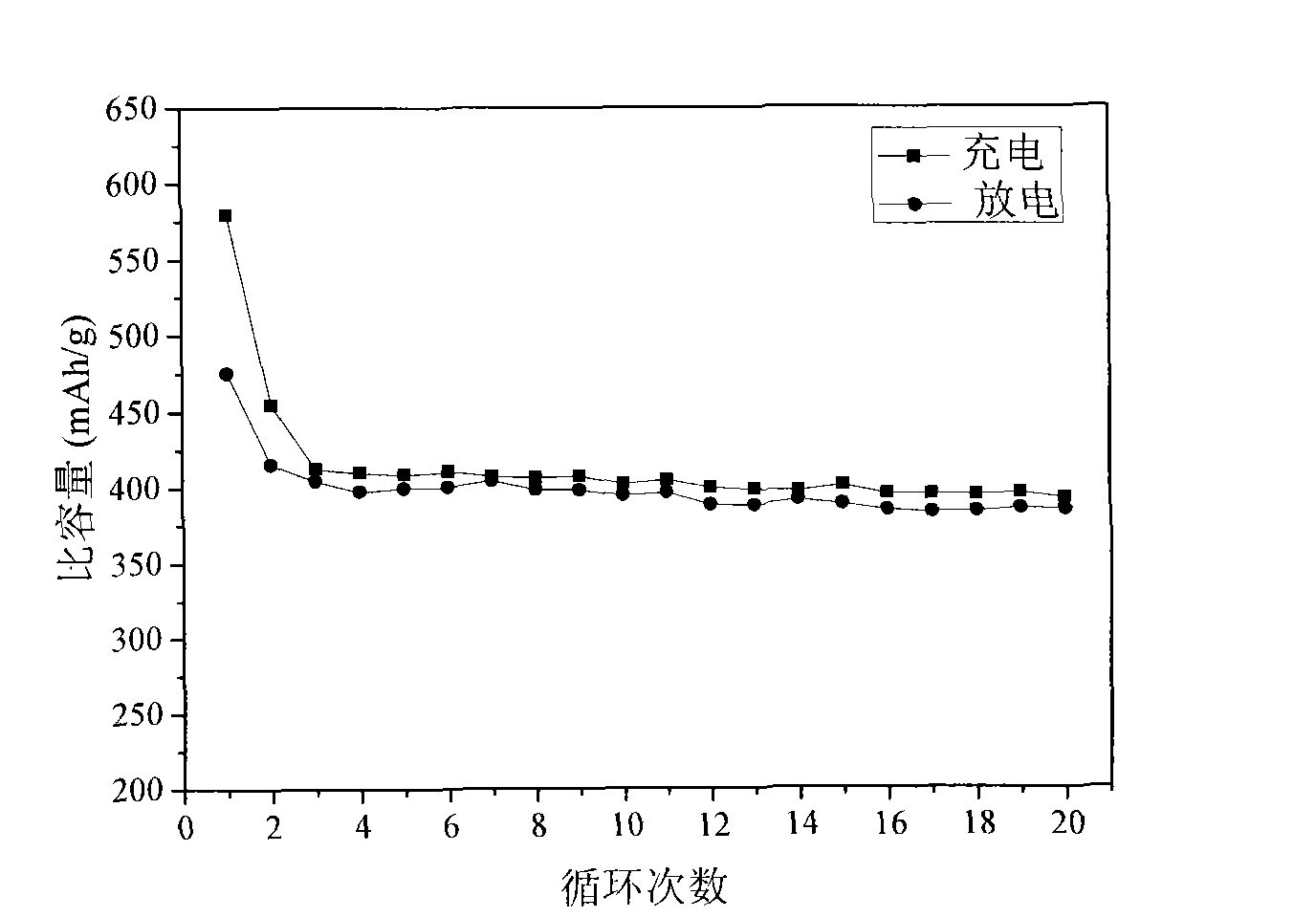
[0031] The prepar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com