Amido metal iridium complex electrophosphorescent luminescent material adopting phenylpyrazole main ligand and preparation thereof
A luminescent material and electrophosphorescence technology, applied in the field of amide-based metal iridium complexes and amide-based metal iridium organic complexes electrophosphorescence luminescent materials, can solve the problems of thermal decomposition, poor anti-crystallization performance, etc., and achieve easy synthesis, The effect of low cost, strong phosphorescent luminescence properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
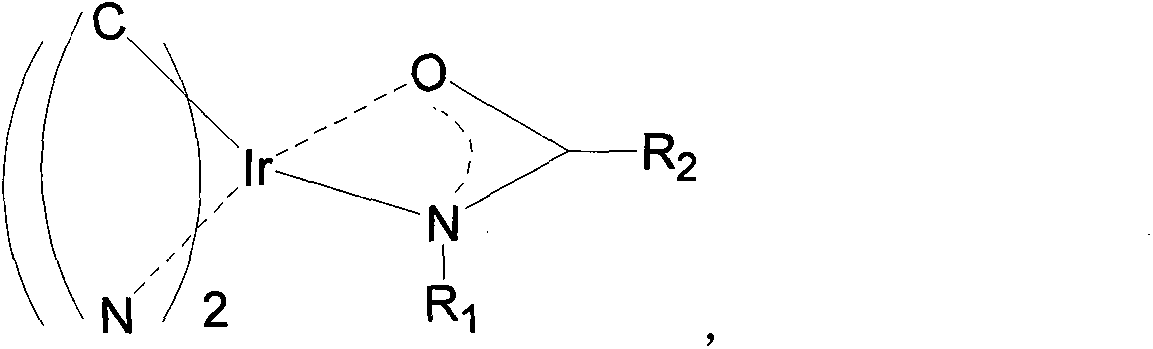
[0027] The preparation method of the metal iridium complex of the present invention: first use aniline or aniline derivatives and acid anhydrides or acid chlorides to heat and reflux in acetic acid to obtain amides (Hayl), and then use the obtained amides (Hayl) and 1-phenylpyrazole derivatives Dichloro bridge compound (C^N) of compound (C^N) 2 Ir(μ-Cl) 2 Ir(C^N) 2 Carry out reaction, obtain target product (C^N) 2 Ir(ayl). Concrete reaction steps are as follows:
[0028] (1) Synthesis of auxiliary ligand (Hayl)
[0029] The synthesis of acetaniline: the mixture of aniline (or 4-methylaniline, 2,6-dimethylaniline, 4-chloroaniline) and acetic anhydride (molar ratio of 1:2) was refluxed in acetic acid for 4 hours, and then The solution obtained by the reaction was poured into ice water, a large amount of solids were precipitated, stirred vigorously for 15 minutes and then suction filtered, and the obtained solids were recrystallized with ethanol to obtain relatively pure ace...
Embodiment 1
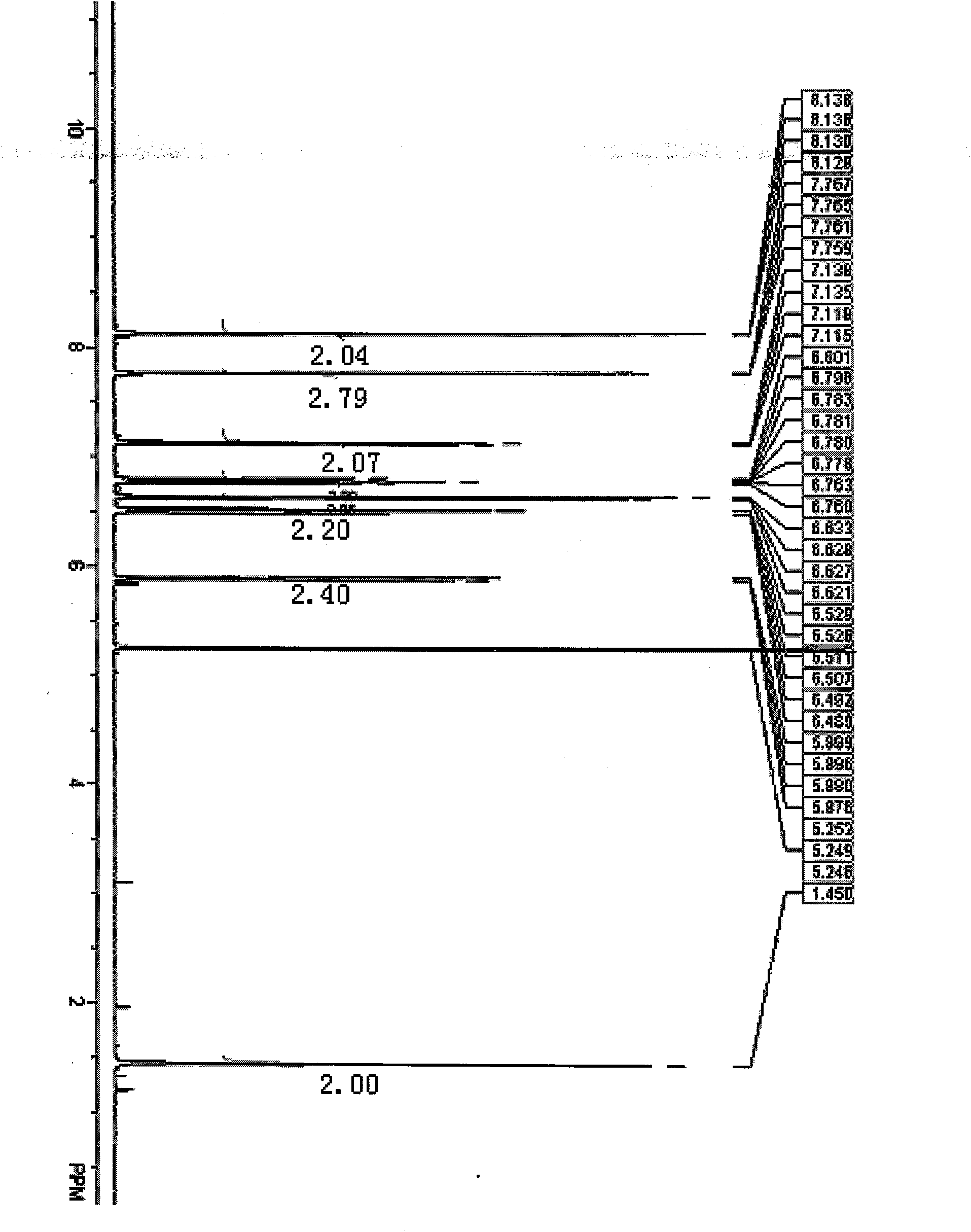
[0036] Embodiment 1: the auxiliary ligand is acetanilide, and the main ligand is the metal iridium complex (C^N) of 1-phenylpyrazole 2 The synthesis of Ir (ayl), i.e. two (1-phenylpyrazole)-acetanilide metal iridium complex, its structural formula is as follows:
[0037]
[0038] (1) Preparation of Acetanilide:
[0039] Add 9.3g of freshly distilled aniline and 20mL of acetic acid into a 100mL round bottom flask, then add 5.1g of acetic anhydride dropwise under stirring, heat to reflux at 120°C, and stop reflux after reflux for 4 hours. After cooling, the reaction solution was poured into ice water, a large amount of white solid was precipitated, filtered with suction, and the obtained white solid was dissolved in 100 mL of ethanol to crystallize. 10.9 g (81.0% yield) of colorless flaky crystals were obtained, m.p=125°C.
[0040] (2) Dichloro bridge compound (ppz) 2 Ir(μ-Cl) 2 Ir(ppz) 2 Synthesis:
[0041] Under nitrogen protection, add 0.068g (0.1929mmol) IrCl to the...
Embodiment 2
[0046] Embodiment 2: the auxiliary ligand is benzoyl naphthylamine, and the main ligand is the metal iridium complex (C^N) of 1-phenylpyrazole 2 The synthesis of Ir (ayl), i.e. two (1-phenylpyrazole)-benzoyl naphthylamine metal iridium complex, its structural formula is as follows:
[0047]
[0048] (1) preparation of benzoyl naphthylamine:
[0049] Add 5g of benzoic acid and 15mL of thionyl chloride into a 100mL round bottom flask, add 2 drops of N,N-dimethylformamide as a catalyst, and introduce the tail gas (HCl) generated by the reaction into a saturated sodium hydroxide solution, and reflux until hydrogen chloride-free generate. Distill under reduced pressure to remove unreacted thionyl chloride to obtain a colorless benzoyl chloride liquid.
[0050] Take another 100mL round-bottomed flask, add 5mL aniline and 30mL chloroform, then dissolve the benzoyl chloride obtained in the previous step in 20mL chloroform, and slowly add it dropwise to the vigorously stirred trie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com