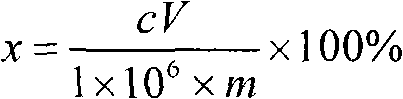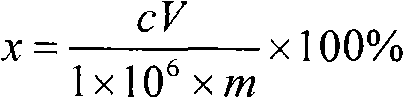Method for rapidly and accurately determining sulphur element content in fluorite
An accurate determination of sulfur element technology, applied in the field of analytical chemistry, can solve problems such as spectral background interference, spectral line interference, and incomplete sample decomposition, and achieve the effects of shortening analysis time, high sensitivity, and reducing detection costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Dry the fluorite sample at 105-110°C for 3-5 hours in advance, put it in a desiccator and cool it to room temperature; weigh 2.0000g of fluorite sample 1# into a 200mL polytetrafluoroethylene beaker, add 30mL deionized water After wetting, add 5mL of 47% hydrofluoric acid, 10mL of 69% nitric acid, 20mL of 38% hydrochloric acid, and 8mL of 72% perchloric acid, heat and dissolve at 300°C, blow perchloric acid fumes until the volume of the solution is 1mL, and make fluorite The sample is completely converted into easily soluble salts; add 50mL of deionized water, then add 10mL of 38% hydrochloric acid, heat to dissolve the salts, after cooling, transfer to a 100mL volumetric flask, dilute to the scale of 100mL with deionized water, and mix well (above The concentration of each acid is the volume percent concentration, the same below). Other steps are the same as in Example 1.
[0052]Treat the blank sample in the same way, add the same concentration of calcium matrix as i...
Embodiment 2
[0057] Weigh 0.2000g of fluorite sample 2# into a 200mL polytetrafluoroethylene beaker, moisten it with a small amount of water, add 2mL of 47% hydrofluoric acid, 5mL of 69% nitric acid, 10mL of 37% hydrochloric acid, and 5mL of 70% perchloric acid. Heat to dissolve at ℃, smoke perchloric acid until the volume of the solution is 0.5mL, so that the fluorite sample is completely converted into easily soluble salts; add 50mL of deionized water, then add 5mL of 37% hydrochloric acid, heat to dissolve the salts, cool Afterwards, transfer to a 100mL volumetric flask and dilute to 100mL with deionized water.
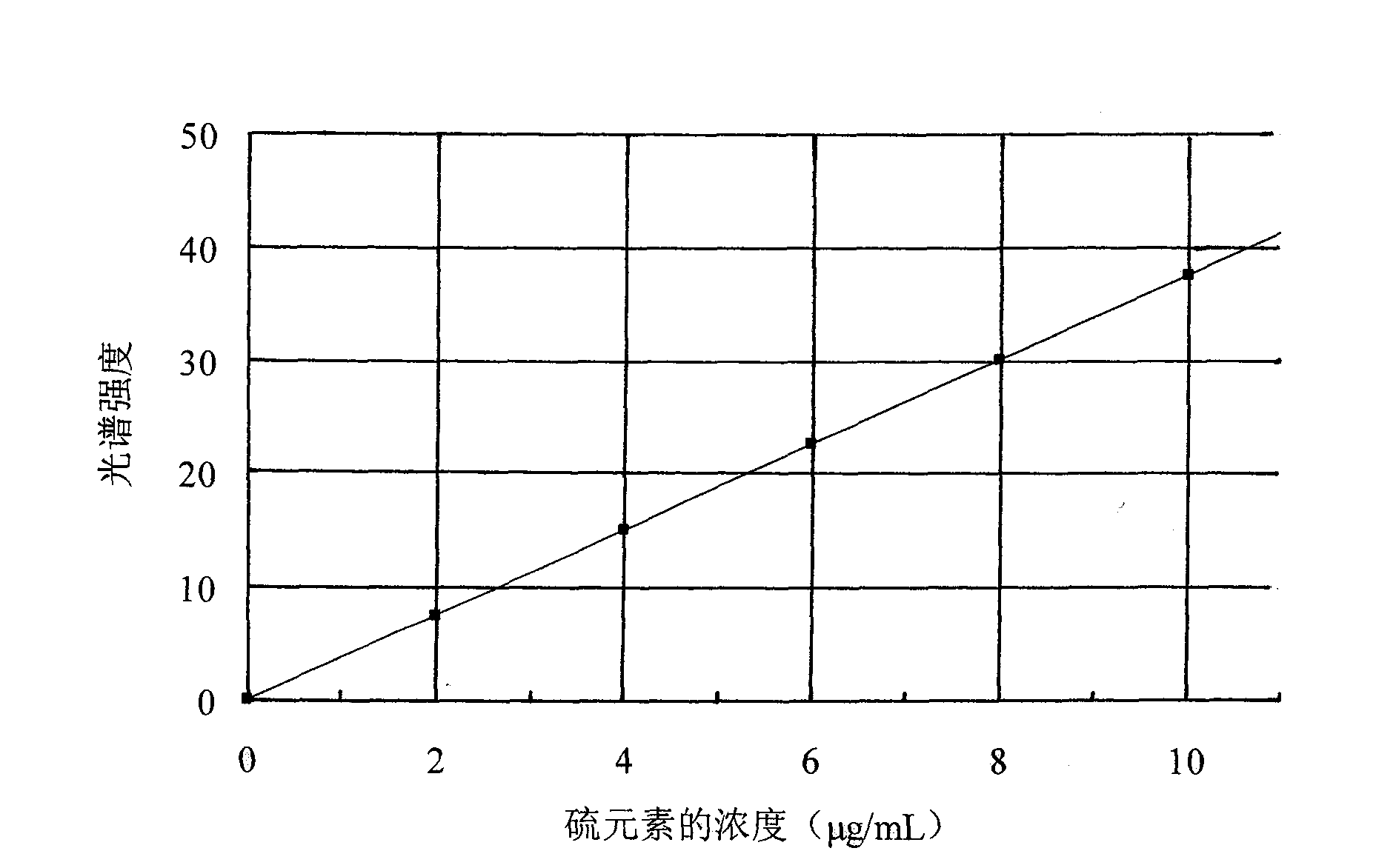
[0058] Treat the blank sample in the same way, add the same concentration of calcium matrix as in the sample, add sulfur standard solutions of different concentrations, add water to make up to 100mL, measure the spectral intensity of sulfur on the ICP emission spectrometer, and draw the working curve. Then measure the spectral intensity of sulfur in the solution to be tested, o...
Embodiment 3
[0063] Weigh 1.0000g of fluorite sample 3# into a 200mL polytetrafluoroethylene beaker, add 20mL deionized water, then add 3mL of 47% hydrofluoric acid, 7mL of 69% nitric acid, 15mL of 38% hydrochloric acid, and 7mL of 72% perchloric acid , heat to dissolve at 250°C, smoke perchloric acid until the volume of the solution is 0.7mL, so that the fluorite sample is completely converted into easily soluble salts; add 50mL of deionized water, then add 7mL of 37% hydrochloric acid, and heat to dissolve the salt Classes, after cooling, transferred to a 100mL volumetric flask, diluted with deionized water to the scale of 100mL.
[0064] Treat the blank sample in the same way, add the same concentration of calcium matrix as in the sample, add sulfur standard solutions of different concentrations, add water to make up to 100mL, measure the spectral intensity of sulfur on the ICP emission spectrometer, and draw the working curve. Then measure the spectral intensity of sulfur in the soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com