Preparation method of ligustrazine phosphate powder injection
A technology of ligustrazine phosphate and ligustrazine powder, which is applied to medical preparations containing active ingredients, powder delivery, pharmaceutical formulations, etc., can solve the problems of low bioavailability, long production cycle, and many degradation products, and achieve the preparation method Simple, short production cycle, less degradation products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
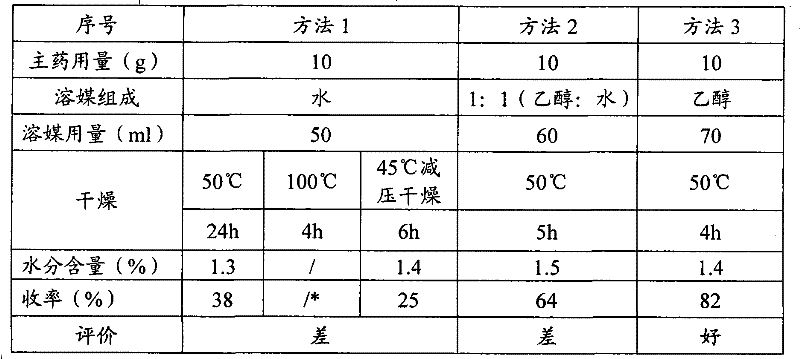
Image
Examples
Embodiment 1
[0030] Dissolve 100g of ligustrazine phosphate in ethanol, heat in a water bath at 60°C to aid dissolution, and prepare 1000mL of ligustrazine phosphate ethanol solution, add 1g of medicinal charcoal to absorb for 20 minutes, filter and decarbonize to obtain the filtrate, and filter the filtrate with a pore size of φ0. 22μm microporous filter membrane, after sterilizing and filtering, crystallize at 0°C for 5 hours, crystallize while stirring at a stirring rate of 1200 rpm, and dry the precipitated crystals at 50°C for 4 hours after filtration to obtain 98 grams of injection Ligustrazine phosphate was used to subpackage, stopper, and cap according to the marked amount of 0.1 g / bottle to prepare 980 ligustrazine phosphate powder injections for injection.
Embodiment 2
[0032] Dissolve 100g of ligustrazine phosphate in ethanol, heat it in a water bath at 60°C to help dissolve it, and prepare 1000mL of ligustrazine phosphate ethanol solution, add 5g of medicinal charcoal to absorb for 50 minutes, filter and decarbonize to obtain the filtrate, and filter the filtrate with a pore size of φ0. After sterilizing and filtering with a microporous membrane of 8 μm, crystallize at 35°C for 12 hours, crystallize while stirring at a stirring rate of 60 rpm, and dry the precipitated crystals at 0°C for 12 hours after filtration to obtain 96.2 grams of injection Ligustrazine phosphate was used to subpackage, stopper, and cap according to the marked amount of 0.1 g / bottle to prepare 962 ligustrazine phosphate powder injections for injection.
Embodiment 3
[0034] Dissolve 100g of ligustrazine phosphate in ethanol, heat it in a water bath at 60°C to help dissolve, and prepare 1000mL of ligustrazine phosphate ethanol solution, add 30g of medical charcoal to absorb for 120 minutes, filter and decarbonize to obtain the filtrate, and filter the filtrate with a pore size of φ0. 45 μm microporous filter membrane, after sterilizing and filtering, crystallize at 20°C for 48 hours, crystallize while stirring at a stirring rate of 2000 rpm, and dry the precipitated crystals at 80°C for 8 hours after filtration to obtain 95.2 g injection Ligustrazine phosphate was used to subpackage, stopper, and cap according to the marked amount of 0.1 g / bottle to prepare 952 ligustrazine phosphate powder injections for injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com