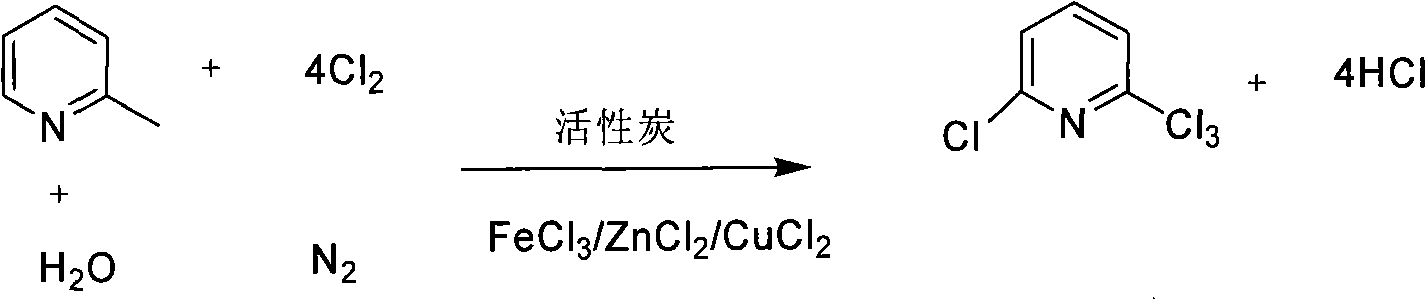
Preparation method of 2- chloro-6-trichloromethyl pyridine
A technology of trichloromethylpyridine and picoline, which is applied in the field of chemical preparation of 2-chloro-6-trichloromethylpyridine, can solve the problem of environmentally unfriendly carbon tetrachloride, lack of market competitiveness, and low reaction yield Unsatisfactory problems, to achieve the effect of low cost, easy operation, and improved selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The method for synthesizing 2-chloro-6-trichloromethylpyridine in this example is mainly to use gaseous 2-picoline and water mixed gas as raw material, nitrogen as carrier gas, and chlorine gas to pass through a fixed bed loaded with catalyst Equipment for continuous reaction. The first step of the reaction operation is the activation of the catalyst. Mix ferric chloride and activated carbon according to the weight ratio of 1:99 and place them on a fixed bed. Turn on the heating system to raise the temperature of the catalyst and keep it warm to 290°C. After activating it with nitrogen at a rate of 0.6L per second for 30 minutes, stop the nitrogen to change the temperature. Feed in chlorine gas and activate at a rate of 0.9L per second for 1 hour. After the catalyst is activated, cool down the fixed bed equipment to 250°C to prepare for chlorination reaction. 200 g of 2-picoline (2.13 mol, content 99%) and 383 g of deionized water (21.3 mol) were added into a mixer for...
Embodiment 2
[0028] The method for synthesizing 2-chloro-6-trichloromethylpyridine in this example is basically the same as the method and reaction steps in Example 1, using a fixed-bed continuous reaction. The catalyst was changed to zinc chloride and activated carbon were mixed according to the weight ratio of 3:17, the introduction amount of 2-picoline and water in the reaction ratio was changed to 15:1mol, and the fixed bed reaction temperature was also adjusted to 280°C. 200 g of 2-picoline (2.13 mol, content 99%) and 575 g of deionized water (31.95 mol) were added to the mixture and mixed for reaction. Turn on the fixed bed tail gas absorption system, keep nitrogen at a rate of 0.5L per second, and chlorine gas at a rate of 0.8L per second through the preheater when the temperature reaches above 150°C, then connect to the fixed bed reaction equipment. The mixed 2-picoline aqueous solution is heated by the vaporizer and enters the fixed-bed reaction equipment in a completely vaporized...
Embodiment 3
[0030] The method for synthesizing 2-chloro-6-trichloromethylpyridine in this example is basically the same as the method and reaction steps in Example 1, using a fixed-bed continuous reaction. The catalyst is changed to cupric chloride and activated carbon is mixed according to the weight ratio of 3:7, the introduction amount of 2-picoline and water in the reaction ratio is changed to 15:1mol, and the fixed bed reaction temperature is also adjusted to 300°C. 200 g of 2-picoline (2.13 mol, content 99%) and 575 g of deionized water (31.95 mol) were added to the mixture and mixed for reaction. Turn on the fixed bed tail gas absorption system, keep nitrogen at a rate of 0.55L per second and chlorine at a rate of 1.0L per second through the preheater when the temperature reaches 150°C or more, then connect to the fixed bed reaction equipment. The mixed 2-picoline aqueous solution is heated by the vaporizer and enters the fixed-bed reaction equipment in a completely vaporized manne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com