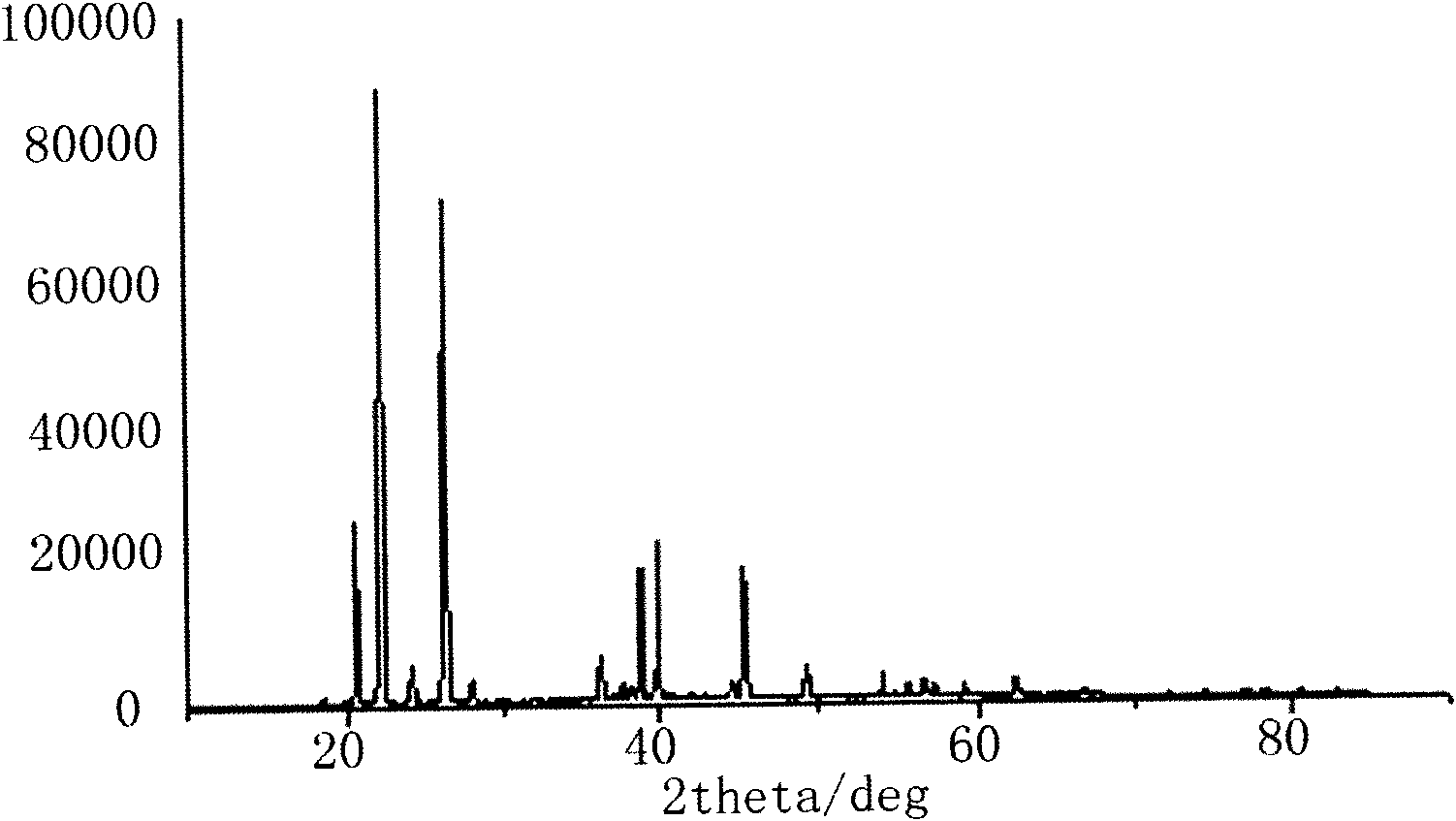
Synthesizing process for obtaining lithium difluoro-oxalato-borate and lithium tetrafluoroborate
A technology of lithium difluorooxalate borate and lithium tetrafluoroborate, which is applied in the field of synthesis technology for obtaining lithium difluorooxalate borate and lithium tetrafluoroborate at the same time, can solve the problems of high equipment requirements and unfavorable industrial production, and achieve the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
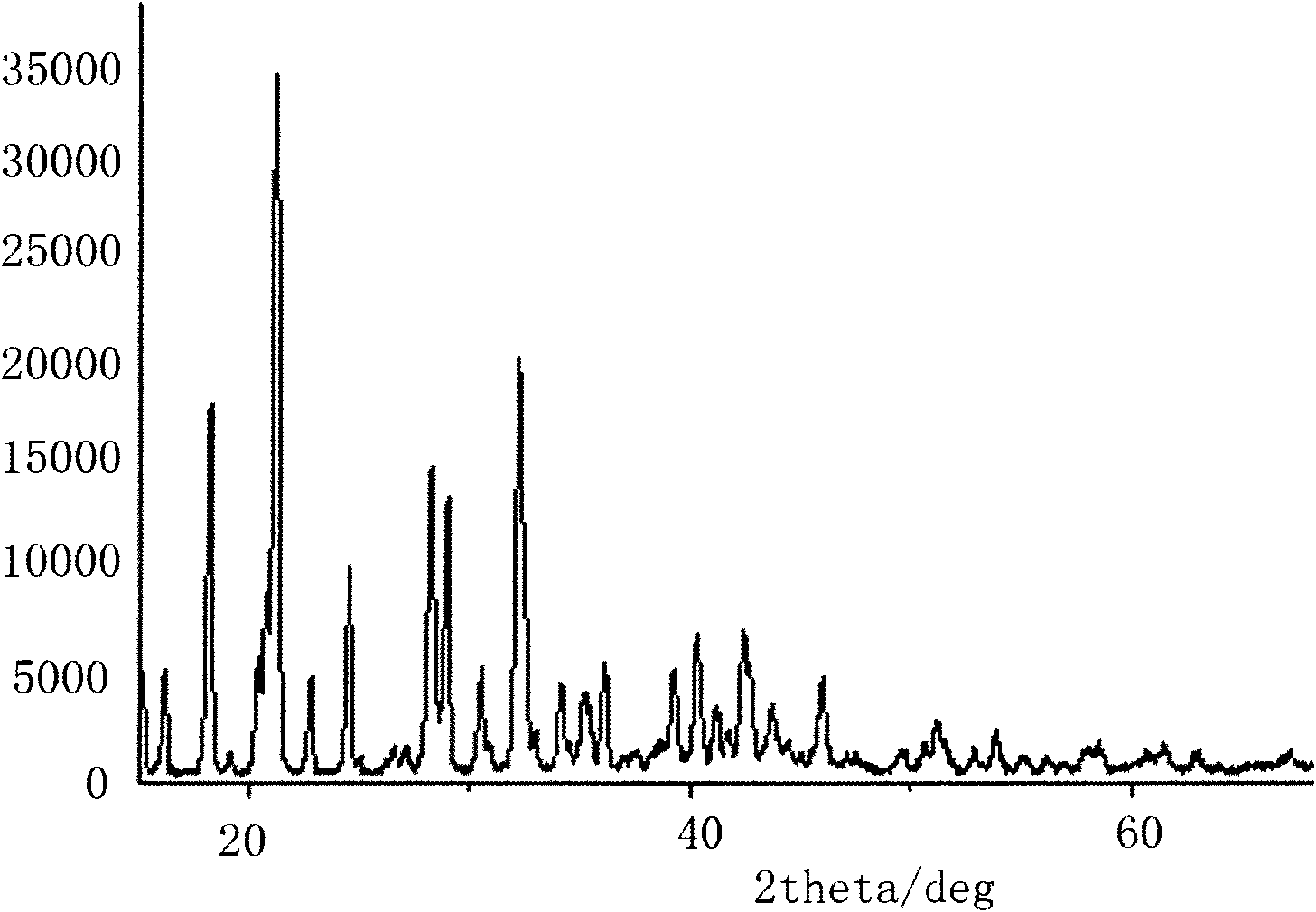
[0022] Using boron trifluoride ether solution and lithium oxalate as raw materials to prepare lithium difluorooxalate borate and lithium tetrafluoroborate.
[0023] Step 1. Add 500g of diethyl carbonate and 101.9g of lithium oxalate dried at 150°C for 10 hours into a dry reaction vessel equipped with a magnetic stirrer and a thermometer, stir to form an emulsion, slowly add 286g of boron trifluoride ether, and react The temperature was adjusted to 80° C. for 24 hours to react to form lithium difluorooxalate borate and lithium tetrafluoroborate, and then cooled to room temperature.
[0024] Step 2: Filtrate to obtain solid lithium difluorooxalate borate and a filtrate containing lithium tetrafluoroborate, extract the solid lithium difluorooxalate borate with ethyl acetate, concentrate under reduced pressure with ethyl acetate, cool and crystallize with ethyl acetate, and recrystallize. At the same time, the filtrate containing lithium tetrafluoroborate was concentrated under re...
Embodiment 2
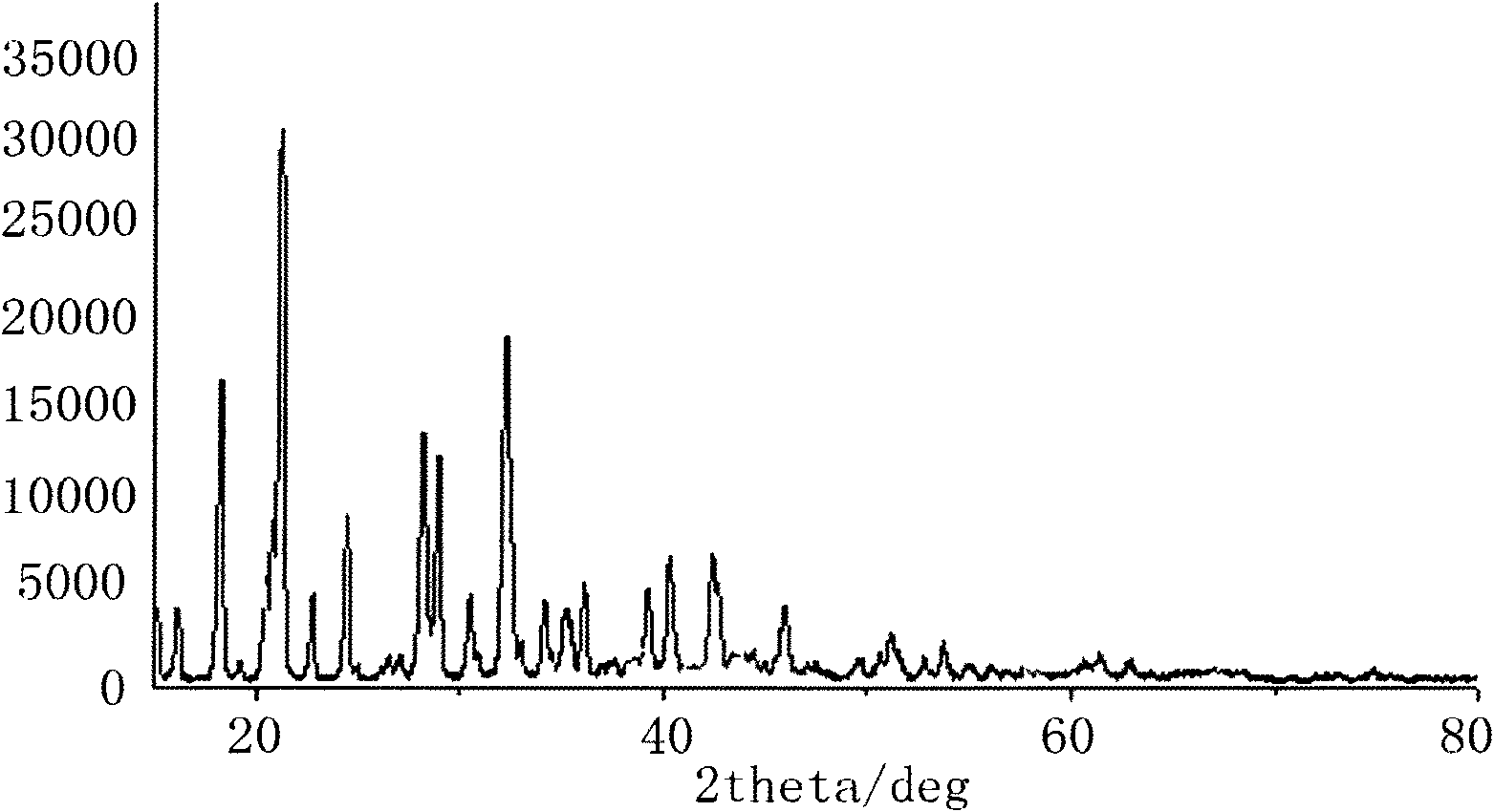
[0028] Using boric acid, oxalic acid, hydrofluoric acid and lithium carbonate as raw materials to prepare lithium difluorooxalate borate and lithium tetrafluoroborate.
[0029] Step 1. Add 300g of hydrofluoric acid (weight concentration: 48%), 123g of boric acid and 90g of oxalic acid in a tetrafluoro reactor equipped with a magnetic stirrer, and control the temperature at about 0°C. After stirring until all are dissolved, slowly add 125g of carbonic acid Stir the lithium to react and release gas while keeping the temperature below 10°C, and continue stirring for 3 hours after no gas is released.
[0030] Step 2, filtering off unreacted lithium carbonate and lithium fluoride, and evaporating the solvent from the filtrate at 0.1Mpa to obtain a white solid. Dissolve the white solid with dimethyl carbonate to a light yellow clear liquid, concentrate and filter to obtain a dimethyl carbonate solution of lithium difluorooxalate borate crystals and lithium tetrafluoroborate. Extrac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com