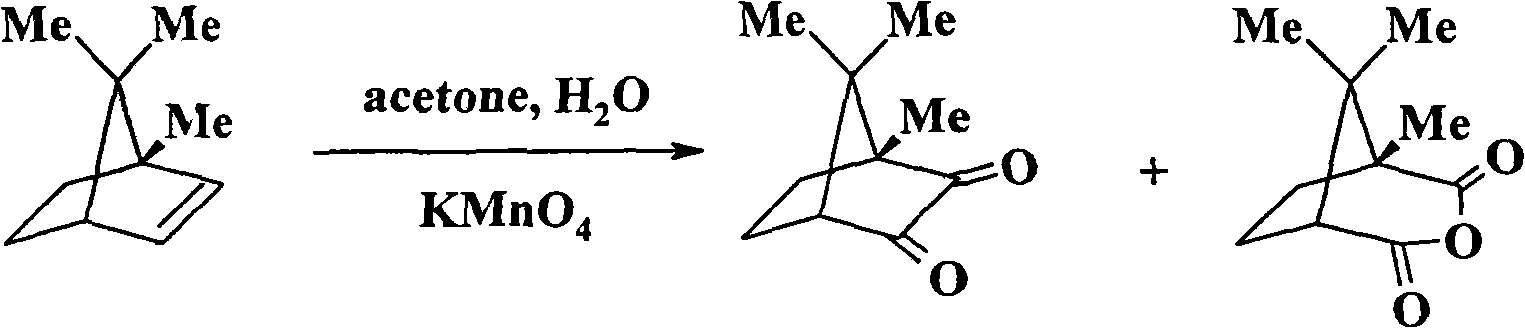
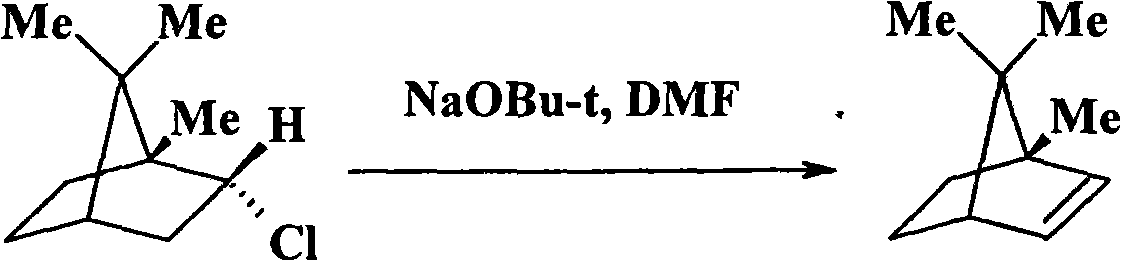Synthetic method of camphorquinone
A synthetic method and technology of camphorquinone, applied in the field of camphorquinone synthesis, can solve problems such as high cost and complex process, achieve low production cost, simplify synthetic route, and broaden the application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
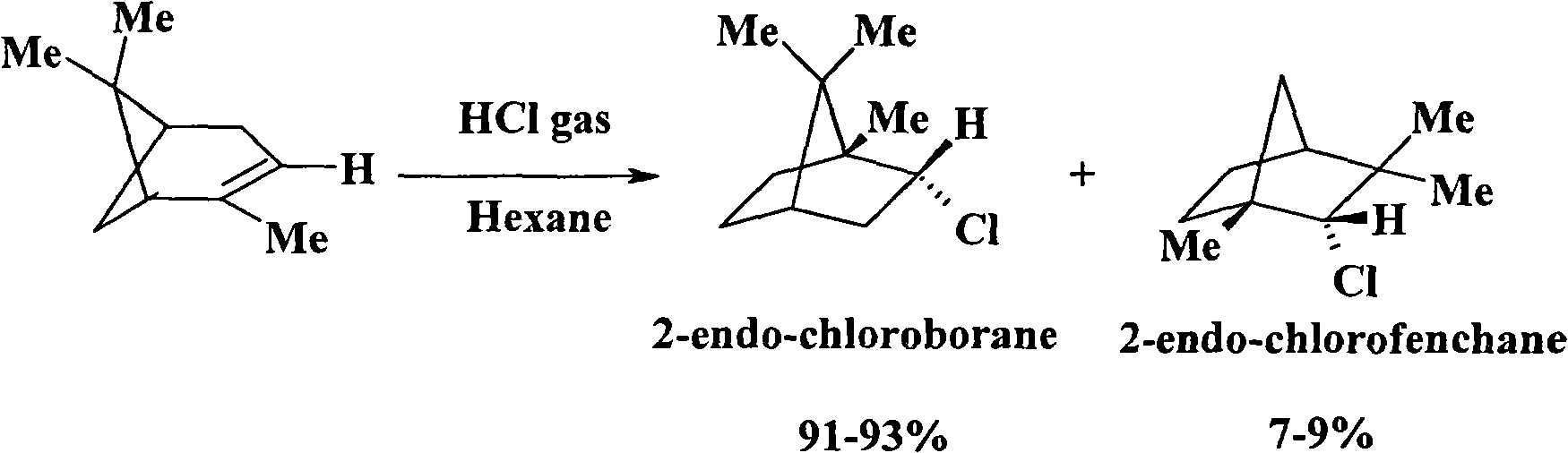
[0039] Add 28g (0.2mole) α-pinene and 50ml n-hexane into a 250ml four-neck flask equipped with a thermometer, stirrer, drying tube and HCl absorption device, and cool the system with an ice-salt bath until the system temperature is -5 to 0°C . Weigh 117g (2mole) of sodium chloride and place it in a 250ml three-necked flask, drop 53.3ml (1mole) of concentrated sulfuric acid into it with a constant pressure dropping funnel, and pass the generated HCl gas into α-pinene after being dried with anhydrous chlorine CaCl In n-hexane solution, react for about 8 hours until HCl gas is no longer absorbed. The resulting reaction solution was washed with 10% NaHCO3 solution to remove excess HCl, the organic layer was dried over anhydrous Na2SO4, and n-hexane was evaporated under normal pressure to obtain solid crude 2-chlorobornane. Recrystallized with absolute ethanol (crude product: ethanol = 1:8-10g / ml) to obtain 31.7g of intermediate 2-chlorobornane with a melting point of 129-131°C an...
Embodiment 2
[0043] Add 28g (0.2mole) α-pinene and 50ml n-hexanepentane into a 250ml four-necked flask equipped with a thermometer, a stirrer, a drying tube and an HCl absorption device, and feed dry HCl gas at room temperature for 6 hours. The resulting reaction solution was treated with 10% NaHCO 3 solution washing to remove excess HCl, and the organic layer was washed with anhydrous Na 2 SO 4 After drying and distilling off n-pentane under normal pressure, the solid crude product 2-chlorobornane was obtained. Recrystallization was performed with a small amount of n-hexane to obtain 20.5 g of intermediate 2-chlorobornane. Dissolve 25.2g (0.2 mole) of potassium n-pentoxide in 100ml of DMF, heat to 135°C, and drop into the 2-chlorobornane-DMF solution with a dropping funnel (17.3g (0.1mole) of 2-chlorobornane is dissolved in 50ml DMF), reacted at this temperature for 4h, after the reactant was cooled, add 100ml of water, extracted 3 times with 100ml of n-hexane, the extract was washed w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com